ABSTRACT
Renal cancer carcinoma is widely considered as one of the most complex and complicated human tumors, due to its variety in presentation, as well as its dangerous aggression. The situation becomes understandably worse after the development of metastatic sites in surrounding organs. The most common “destination” for such malignancy is the pancreas. In this report, we depict two patients who presented with metastatic renal carcinoma on their pancreas. The first case describes the condition of a patient, who underwent distal pancreatectomy with splenectomy, after the identification of two metastatic sites (on the tail and head). The second case concerns a patient, who presented with a solitary metastatic tumor on the body of the pancreas. Distal pancreatectomy with splenectomy was decided as the treatment option. Unfortunately, the presence of renal cancer metastatic sites makes the survival rates significantly lower. Thus, diagnostic methods need to be as fast and effective as possible, so as to plan an efficient therapeutic method without any delay.
INTRODUCTION
Renal cancer refers to a tumorous cell growth inside the renal parenchyma. In general, its etiology remains relatively unknown and controversial, while its risk factors are more prominent. These include tobacco use, chronic kidney disease, physical condition and presence of hypertension.[1] Renal cancer can be present in many different forms, but clear cell renal cancer is the most common type, accounting for nearly 80% of all malignant tumors located in the kidney. Moreover, it should be noted that this kind of aggressive cell mass is capable of causing a variety of paraneoplastic manifestations so as to mimic the presence and characteristics of other tumors.
Clear cell renal cancer belongs to the broad category of renal cell carcinoma, which comprises the majority of kidney tumors. This extensive group of kidney cancer can be divided into 3 main categories, according to traditional morphological criteria. Precisely, papillary, chromophobe and clear cell are the three main subgroups.[2] Clear cell carcinoma remains the most frequent metastatic tumor to the pancreas. Two cases of pancreatic resection for metastatic renal carcinoma to the pancreas are presented in addition to literature review. Each cancer category has different percentages for distant metastases, resulting in varying levels of aggression.
In general, incidence rates of renal cancer increase as the age of the person increases, while it is a more common finding in male patients than female ones.[3]
CASE PRESENTATION
The first case report describes a 67-year-old male patient who underwent peripheral pancreatectomy after being diagnosed with metastatic renal cancer on the tail of the pancreas in addition to a small lesion in the pancreatic head. A right nephrectomy had been performed in 2007. Distal pancreatectomy with splenectomy was performed, while splenic vessel ligation at the origin was demonstrated in addition to lymph node dissection. The cross sections from the body and the tail of the pancreas revealed a grey-brown epicenter, with a size of 0.8 cm. As far as the microscopic examination is concerned, development of a tumorous mass is reported. Certain immunomorphological markers support the presence of metastasis from renal cell carcinoma. Specifically, the immuno phenotype was AE1/AE2+, CK7-, CD10+, Vim+, CK20-, RCC slightly+. This carcinoma was developing inside the parenchyma, without spreading towards the surfaces of the surgical specimen. It is important to note that six lymph nodes, without any cancerous infiltration, were found in peri-pancreatic fatty tissue. Intense epicenters of enzymic-type necrosis were identified in the rest of the pancreatic parenchyma. Apart from necrosis, the rest of the parenchyma presented with PanIN1β and small cystic areas that are coated with non-stacked mucous epithelium, without any nuclear atypia. Thus, this case represents two hyper-vascular lesions, which were located on the head and the body of the pancreas. The decision regarding the patient’s treatment was the performance of distal pancreatectomy with splenectomy, as well as arterial embolization of the pancreatic head lesion, which showed complete response. The patient remained alive without recurrence 8 years postoperatively (Figures 1–3).
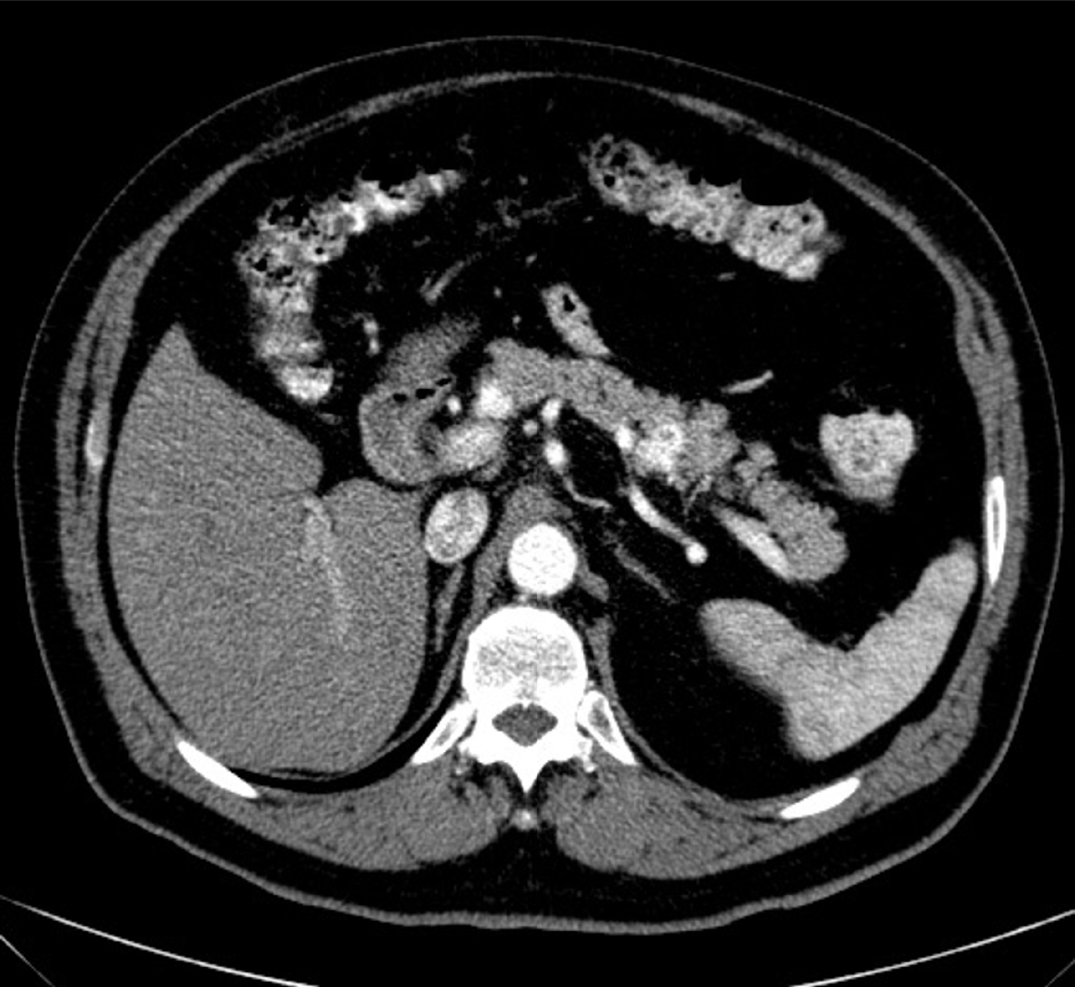
Figure 1:
CT scan showing a solitary metastatic tumor i the tail of the pancreas.
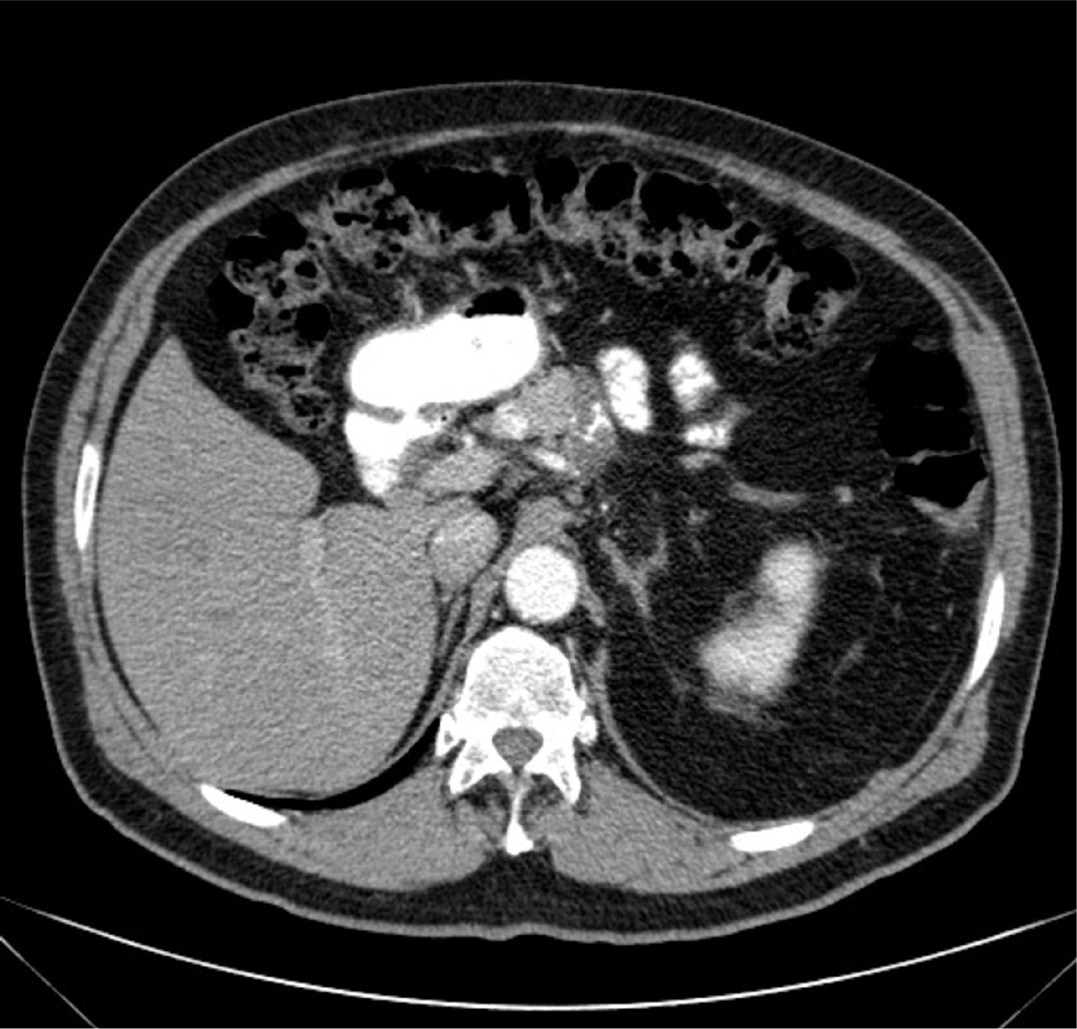
Figure 2:
Axial CT scan of the abdomen.
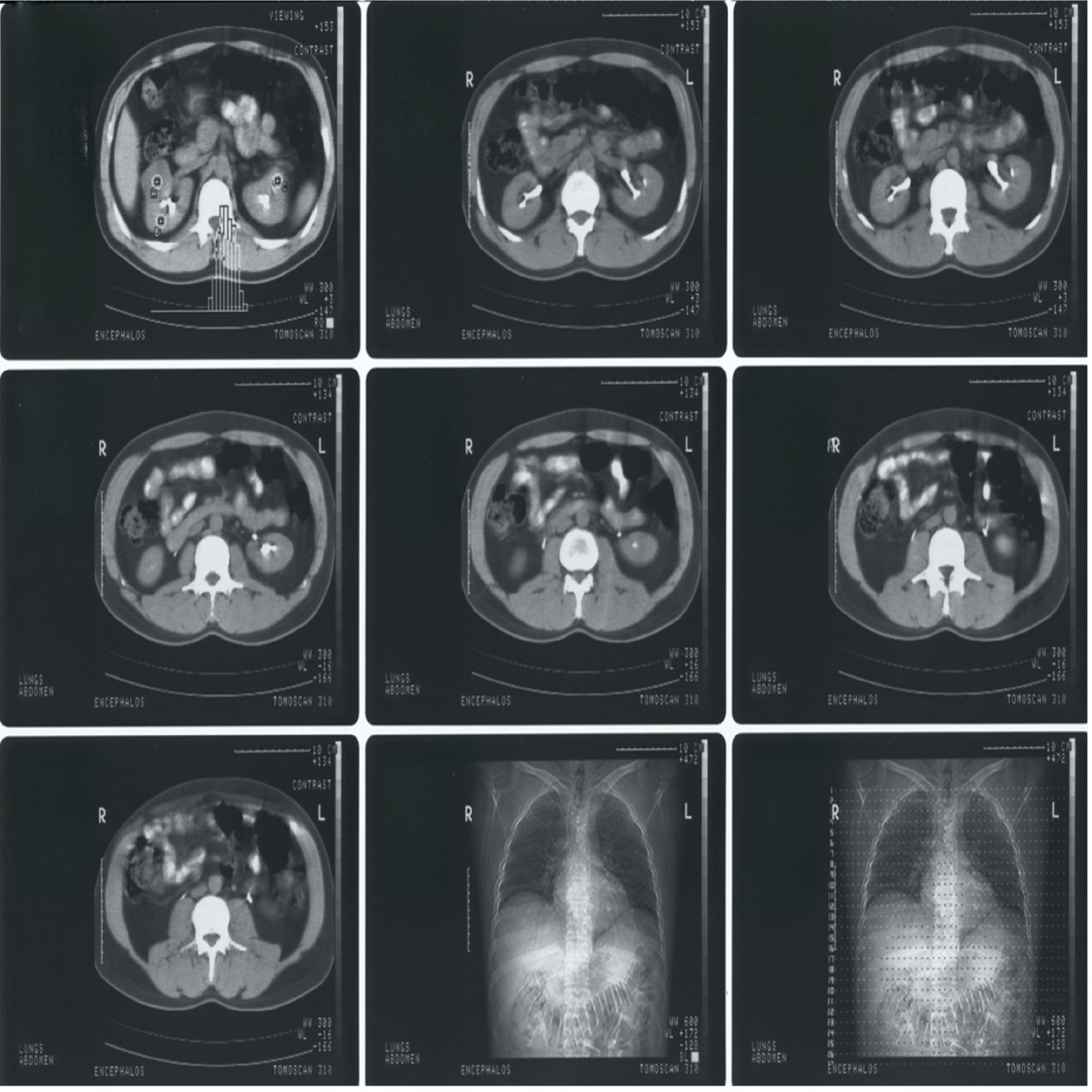
Figure 3:
First row depicts CT scans of the abdomen, highlighting the pancreas, second row includes CT scans, depicting the metastatic sites on the pancreas, and the third row contains chest X-rays that confirm that no metastatic sites are present in the lungs.
The second case report concerns a 56-year-old patient who also presented with renal metastatic cancer to the neck of the pancreas. After the execution of cross-sections, four hepatic lymph nodes were revealed, with mean diameters of 0.5-1.2 cm. Pancreatic specimen had a size of 13.4×4.8×2.5 cm with excision boundaries towards the pancreatic neck of 3.3 cm.
Moreover, the spleen, 11.1×7.4×2.7 cm size, as well as the peri-splenic fat, 13.4×6.3×3 cm size, were extracted from the patient. A cross-section was performed 1.5 cm away from the excision boundaries towards the neck and the body of the pancreas. Through this examination, an orange-grey, compact and elastic tumorous mass, with a size of 1.4x1x3x1 cm, was discovered in approximation to the anterior surface of the histological product. It is important to note that in the rest of the pancreatic parenchyma, a “lobe-like” brown-grey pattern was mostly identified. As far as the spleen and peri-splenic fat is concerned, the cross sections depicted a brown-grey homogenous splenic parenchyma, while 12 lymph nodes, with mean diameters of 0.1-0.7 cm, were found inside the fat tissue.
The macroscopic examination of the histological fragments provided significant and helpful information for the final verdict. Precisely, the medical staff observed regional fibrosis and expanded and hyperemic blood vessels inside the mature fat tissue fragments. Additionally, another important highlight is the presence of 4 lymph nodes with slight reactive-type deterioration but no cancerous infiltration.
Taking into consideration the patient’s medical history as well as the immuno morphological markers, the presence of a metastatic «clear-cell” renal cancer seems like a sensible diagnosis. This tumorous mass can be described as well-defined and surrounded by a fibrous “capsule”. It should be noted that it extends outside the surgical surfaces of the specimen. In the rest of the pancreatic parenchyma, rare epicenters of acinar-to-ductal metaplasia, as well as epicenters of enzymatic-type necrosis.
Therefore, in the second medical case, the patient presented with a solitary metastatic tumor on the body of the pancreas, originating from the kidneys. The decision regarding the patient’s treatment was the performance of distal pancreatectomy with splenectomy (Figure 4).
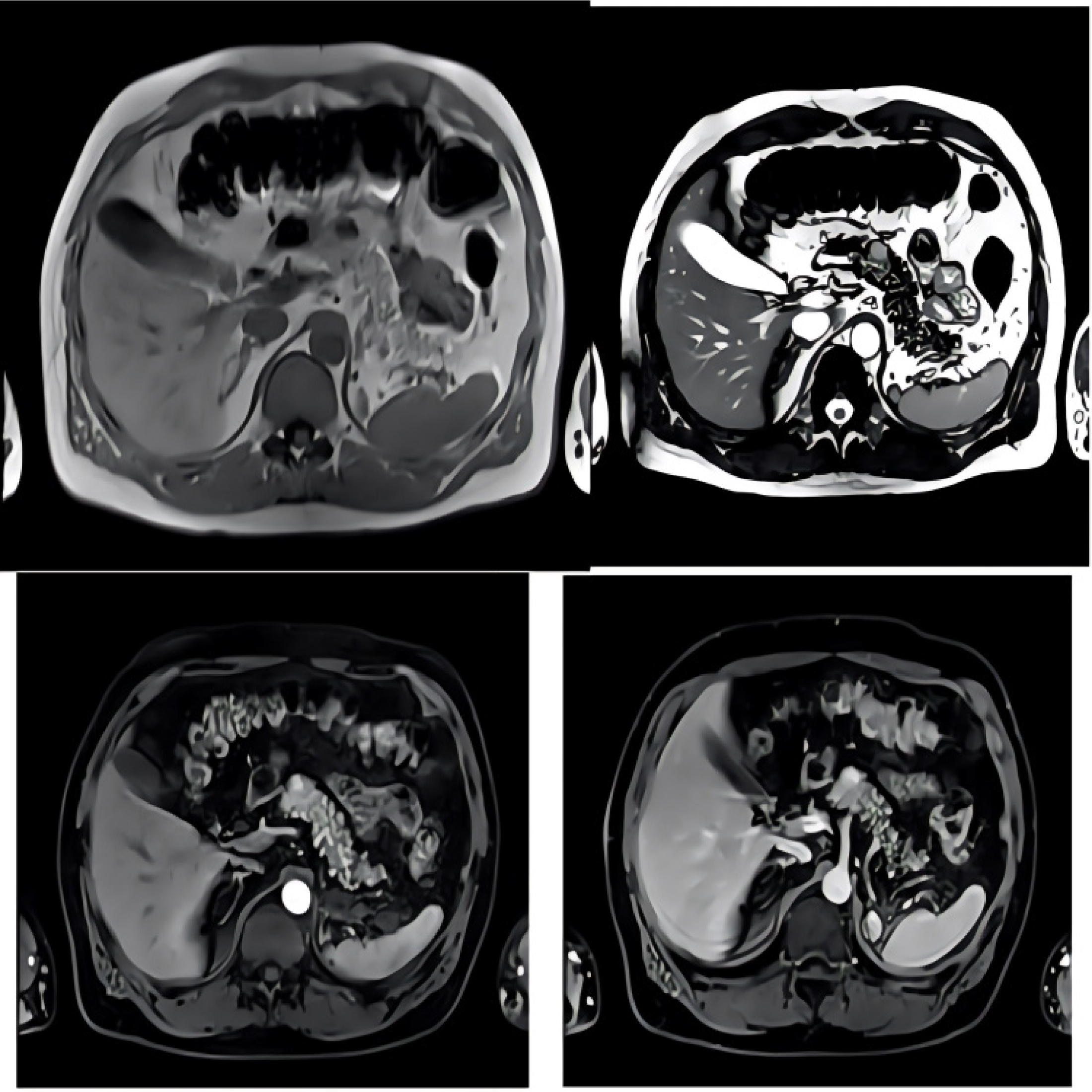
Figure 4:
Axial MRI scan which depicts the metastatic sites from the kidneys to the pancreas.
DISCUSSION
Renal cancer is reported to be among the top 15 most common cancer types worldwide. Even though mortality rates can vary between different countries, the average incidence is approximately 10 cases per 100,000. Fortunately, these statistics have been shown to steadily decline over the years, as a response to the development of high-quality treatment options. As mentioned above, the presence of metastatic sites can change the patient’s survival chances dramatically. Specifically, even though localized tumors can present with a survival rate of up to 90%, distant metastases and limited spread are connected with 12% and 72.5% respectively.[4]
In general, it should be noted that the presence of metastases on the pancreas are considered a rare site, which makes up less than 3% of total pancreatic malignancies.
The severity of such cases is also supported by the fact that any type of tumor is able to present a secondary malignancy on the pancreas. According to autopsy and surgical databases, primary tumors from lymphomas, carcinomas of the stomach, kidney, lung, and GI tract are more commonly responsible for pancreatic metastases.[5] Other scientific reviews that were conducted, presented detailed percentages of the different type of metastatic site origins. Specifically, the most frequent was renal cancer, at 62%, then non-small-cell lung cancer, followed by melanoma. Other, but not so common, malignancies which have been observed to give metastatic sites on the pancreas are thyroid, ovarian, hepatocellular carcinomas, and prostate.[6,7]
It should be remembered that secondary metastatic malignancies on the pancreas can be present with more than one pattern. Precisely, in 5-10% of patients, numerous small nodules, which merge occasionally into a larger mass, can be reported. The depletion of these nodules is heavily dependent on the histological type of the primary tumor. Moreover, another morphological display, present in 15-44% of patients, is observed through a diffuse infiltration of the pancreas, resulting in an indirectly enlarged organ. Finally, the third pattern, found in 50-75% of patients, is described as a singular mass with an infrequent hypodense focus on the contrast-enhanced CT.[8,9]
Diagnosis of dangerous medical entities is always characterized as crucial due to the necessity to manage and treat the abnormality as soon as possible. Thus, diagnosis of metastasis on the pancreas must be executed with carefulness and caution. In general, suspicion arises from the history of a relevant primary cancer. Majority of secondary pancreatic malignancies are observed through a CT, which takes place in patients who undergo follow-up examinations after a primary tumor. Other than the use of CT, dynamic contrast-enhanced CT, Magnetic Resonance Imaging (MRI), contrast-enhanced ultrasonography, and Endoscopic Ultrasonography (EUS), can also be helpful in discovering the metastatic site.[7] Each imaging technique presents with each own advantages, as well as display characteristics, which must be taken into consideration before analyzing each image. Thus, in Ultrasound imaging, the metastatic sites are described as hypoechogenic masses inside the pancreatic parenchyma, while cysts are not really observed. Even after a negative CT scan, EUS may be able to identify and depict the presence of secondary metastatic malignancies on the pancreas. Furthermore, EUS-guided Fine-Needle Aspiration (FNA) is also another possible diagnostic technique, which can prove helpful in locating metastases from different primary tumors.[10] The introduction of a technologically advanced imaging technique, called Contrast-Enhanced Ultrasonography (CEUS), has provided significant advancements in locating pancreatic lesion or characterizing malignancies already noticeable with the US. This imaging method offers better results, when dealing hyper-vascular tumors, such as renal cell cancer, hepatocellular carcinoma, and neuroendocrine tumors.[11] Moving on, MR imaging depicts the lesions as hypointense, when compared with normal gland tissue on T1-weighted images. On the other hand, T2-weighted images display the lesions with heterogenous or relatively hyperintense signal.[7] Appropriate imaging and careful diagnosis is extremely significant in metastases from renal cell carcinomas, since they present with strong enhancement both on the CT and MRI. This observation can be explained through the hypervascularity of the viable tumor tissue, while also being the main differential diagnostic feature from the hypo vascular pancreatic adenocarcinoma. Therefore, in such cases early scanning, after intravenous contrast administration is advised.[12] Despite the fact that solitary metastatic sites from renal cell carcinoma are the most common type, the presence of multifocal lesions is not rare, especially in cases of lung cancer or colonic cancer metastases.
Differential diagnosis is critical when managing such cases, due to the similarities with other independent diseases. Specifically, understanding and identifying the differences between secondary pancreatic tumors and primary pancreatic adenocarcinomas can be challenging. Primarily, the use of CT is not able to identify small differences in attenuation between nonnecrotic or non-cystic neoplastic tissue and normal tissue of the pancreas. This observation is considered critical when dealing with metastatic nodules, which do not modify the physiological contour of the pancreas.[9] Additionally, differentiation between non-hyperfunctioning islet cell carcinoma and other metastatic tumors that do not look similar to the common ductal type of pancreatic adenocarcinoma, can be quite demanding. In cases of renal cell carcinoma, both diseases share a lot of common elements, morphologically as well as in imaging features. Both types of malignancies are characterized as hyper-vascular, while also being subject to central necrosis and cystic degeneration.[7]
Patients may develop epigastric abdominal pain or acute pancreatitis following the ductal obstruction from the metastatic lesion, or be completely asymptomatic. Other clinical signs, such as GI bleeding or painless jaundice after biliary obstruction, can be the result of either metastatic sites or primary pancreatic tumors. In cases where patients present renal cell carcinoma, the triad of flank pain, palpable mass and gross hematuria is a classic symptom that is only present in 10% of newly diagnoses cases.[13]
As far as prognosis of the patient is concerned, statistics heavily depend on a variety of factors that can impact the survival rates. Precisely, the execution of surgical management of solitary pancreatic metastases that originate from malignancies, other that renal cell carcinoma, present with a poor prognosis. Around 2-6% of patients who are reported to have metastatic renal cell carcinoma, are also observed to have isolate metastatic sites, “susceptible” to surgical resection. This type of operation on secondary lesions, limited to the pancreas, is reported to have a 5-year survival rate of 29-35%. Moreover, the presence of an extended waiting period between the appearance of metastatic sites and the implementation of nephrectomy, typically results in a better prognosis. The fact that the involvement of lymph nodes around the pancreas is an uncommon site, radical lymph node dissection can easily be avoided.[14,15]
Treatment and management of pancreatic metastases is widely considered as an interesting medical challenge that requires attention, caution and a multifaceted approach. Precisely, there is not enough confidence in the potential benefit of surgical treatment, whereas surgical resection is considered as a safe option for patients with isolated metastatic sites. Even though long-term survival is not common, surgery should be considered in the multimodality approach to this case.[16] In specific cases of renal cell cancer as the primary tumor, complete resection of the secondary lesions can be achieved with a low complication rate. It should be noted that survival rate is a complicated topic, where not sufficient data are available at the moment. It is thought that the number of metastases, as well as the size of the lesion heavily impact the chances of survival.[7] In patients with advanced renal cell carcinoma, the provision of sunitinib showed promising results, while also being effective in pancreatic metastases. These reports create controversy, when dealing with patients with metastatic renal cell carcinoma on the pancreas, regarding the difference in benefit between anti-angiogenic agents and aggressive surgical resection. Finally, the provision of adjuvant therapy, chemotherapy, immunotherapy and radiation therapy, after the execution of surgical resection, has presented disappointing results. However, new data regarding the benefit of molecular targeted therapy for renal cell carcinoma have been promising.[17]
In a recent study, Duarte et al., combined and analyzed data regarding major characteristics of metastatic renal cell carcinoma. Among the multiple findings, great emphasis should be given on the future of treatment and management methods.
Scientists believe that the key point in developing a new treatment pathway is to take advantage of the metastatic tumor’s needs of an established blood supply. Thus, instead of inhibiting the angiogenesis of the secondary malignancy, an alternative approach is to target the existing tumor blood supply networks. Renal cell carcinoma’s metastatic sites on the pancreas have been reported to present with increased angiogenesis and enhancement for PBRM1 mutations, while also having decreased BAP1 mutations and a physiological stroma.[18] In another study, which examined patients with renal cell carcinoma metastases on multiple locations, the data supported the idea that pancreatic secondary lesions had greater PBRM1 modifications, in comparison to the other types.
The completion of another statistical analysis of 31 cases with renal cell carcinoma metastases to the pancreas depicted that these types of patients have prospective sensitivity to anti-angiogenic therapeutic agents, along with a significant resistance to Immune Checkpoint Inhibitors. Genomic analysis of the secondary malignancies on the pancreas showed augmented rates of PBRM1, ALK, and NTRK3 mutations, while also having decreased rates of BAP1, MTAP, CDKN2A mutations, low PD-L1 positivity.[19]
Therefore, the fact that metastatic sites on the pancreas have a less immunogenic phenotype, it is suggested that the inclusion of VEGFR treatment may be more beneficial compared to Immune-Oncologic therapy.[19]
Our presentation of the two case reports provides critical insight regarding the medical management of two patients who presented with metastatic renal cell carcinoma to the pancreas. As mentioned above, such medical cases are extremely uncommon and dangerous. Thus, the inclusion and description of such instances offers unique and rare medical knowledge regarding the diagnosis, treatment and outcome of these type of patients. The detailed description of the histological examinations, as well as the inclusion of imaging figures, contributes in the better understanding of the morphology, as well as the clinical display of this significant disease.
CONCLUSION
Renal cell cancer is characterized as a very complex and dangerous carcinoma that requires detailed and careful medical approach. Renal cancer can be categorized into many different subtypes according to traditional morphological criteria. Unfortunately, patients may present with metastatic sites, which makes their
survival rates concerningly lower. The utilization of different imaging techniques is clinically significant, so as to be able to identify and plan a treatment plan as fast as possible. Finally, various therapeutic methods can be applied, depending on the unique features of each clinical case.
Cite this article:
Chrysikos D, Delis S, Triantopoulou C, Charitaki E, Kokoroskos N, Taprantzis N, et al. Two Cases of Pancreatic Metastases from Renal Cell Carcinoma and Review of the Literature. Journal of BUON. 2024;27:43-49.
ABBREVIATIONS
| AE1/AE2 | Anion Exchangers |
|---|---|
| CK7 | Cytokeratin 7 |
| CD10 | Cluster of Differentiation |
| Vim | Vimentin |
| CK20 | Cytokeratin 20 |
| RCC | Renal Cell Carcinoma |
| PanIN1β | Pancreatic Intraepithelial Neoplasia |
| CT | ComputedTomography |
| MRI | MagneticResonance Imaging |
| CEUS | Contrast enhanced Ultrasonography |
| EUS | Endoscopic Ultrasonography |
| FNA | Fine-needle Aspiration |
| US | Ultrasonography |
| GI | Gastrointestinal |
| PBRM1 | Polybromo-1 |
| ALK | Anaplastic Lymphoma Kinase |
| NTRK3 | Neurotrophic receptor tyrosine kinase 3 |
| BAP1 | BRCA Associated Protein 1 |
| MTAP | Methylthioadenosine phosphorylase |
| CDKN2A | Cyclin-Dependent Kinase Inhibitor 2A |
| PD-L1 | Programmed death-ligand 1 |
| VEGFR | Vascular Endothelial Growth Factor Receptor. |
References
- Scelo G, Larose TL. Epidemiology and Risk Factors for Kidney Cancer. J Clin Oncol. 2018;36(36):JCO2018791905 [Google Scholar]
- Khandpur U, Haile B, Makary MS. Early-Stage Renal Cell Carcinoma Locoregional Therapies: Current Approaches and Future Directions. Clin Med Insights Oncol. 2024;18:11795549241285390 [Google Scholar]
- Scelo G, Li P, Chanudet E, Muller DC. Variability of Sex Disparities in Cancer Incidence over 30 Years: The Striking Case of Kidney Cancer. Eur Urol Focus. 2018;4(4):586-90. [Google Scholar]
- Padala SA, Barsouk A, Thandra KC, Saginala K, Mohammed A, Vakiti A, et al. Epidemiology of Renal Cell Carcinoma. World J Oncol. 2020;11(3):79-87. [Google Scholar]
- Spadaccini M, Conti Bellocchi MC, Mangiavillano B, Fantin A, Rahal D. Secondary Tumors of the Pancreas: A Multicenter Analysis of Clinicopathological and Endosonographic Features. J Clin Med. 2023;12(8):2829 [Google Scholar]
- Deshpande SS, Joshi AR, Mankar D. Pancreatic Neoplasms: CT Evaluation of the Uncommon Presentations of Common Lesions and Common Presentations of the Uncommon Lesions!. Indian J Radiol Imaging. 2022;32(4):531-9. [Google Scholar]
- Triantopoulou C, Kolliakou E, Karoumpalis I, Yarmenitis S, Dervenis C. Metastatic disease to the pancreas: an imaging challenge. Insights Imaging. 2012;3(2):165-72. [Google Scholar]
- Veron Sanchez A, Santamaria Guinea N, Cayon Somacarrera S, Bennouna I, Pezzullo M, Bali MA, et al. Rare Solid Pancreatic Lesions on Cross-Sectional Imaging. Diagnostics (Basel). 2023;13(16):2719 [Google Scholar]
- Balasubramaniam R, Sammut JS, Britton I. Metastatic small cell lung cancer presenting as acute pancreatitis: Diagnosis with magnetic resonance cholangiopancreatography. Radiol Case Rep. 2020;15(11):2250-4. [Google Scholar]
- Spadaccini M, Conti Bellocchi MC, Mangiavillano B, Fantin A, Rahal D. Secondary Tumors of the Pancreas: A Multicenter Analysis of Clinicopathological and Endosonographic Features. J Clin Med. 2023;12(8):2829 [Google Scholar]
- D’Onofrio M, de Sio I, Mirk P, Vidili G, Bertolotto M, Cantisani V, Schiavone C, et al. SIUMB experts committee. SIUMB recommendations for focal pancreatic lesions. J Ultrasound. 2020;23(4):599-606. [Google Scholar]
- Chin W, Cao L, Liu X, Ye Y, Liu Y, Yu J, et al. Metastatic renal cell carcinoma to the pancreas and subcutaneous tissue 10years after radical nephrectomy: a case report. J Med Case Rep. 2020;14(1):36 [Google Scholar]
- Zhang Y, Li X, Zuo S, Ma X, Chen L, Xiong L, et al. The prognostic value of visible hematuria is only significant in T1a renal cell carcinoma: a single-center retrospective study. BMC Urol. 2024;24(1):247 [PubMed] | [CrossRef] | [Google Scholar]
- Sohn TA, Yeo CJ, Cameron JL, Nakeeb A, Lillemoe KD. Renal cell carcinoma metastatic to the pancreas: results of surgical management. J Gastrointest Surg. 2001;5:346-51. [PubMed] | [CrossRef] | [Google Scholar]
- Thadani A, Pais S, Savino J. Metastasis of renal cell carcinoma to the pancreas 13 years postnenhrectomv. Gastroenterol Hepatol (N Y). 2011;7(10):697-9. [PubMed] | [CrossRef] | [Google Scholar]
- Tani R, Hori T, Yamada M, Yamamoto H, Harada H, Yamamoto M, et al. Metachronous Pancreatic Metastasis from Rectal Cancer that Masqueraded as a Primary Pancreatic Cancer: A Rare and Difficult-to-Diagnose Metastatic Tumor in the Pancreas. Am J Case Rep. 2019;20:1781-7. [PubMed] | [CrossRef] | [Google Scholar]
- Battelli C, Cho DC. mTOR inhibitors in renal cell carcinoma. Therapy. 2011;8(4):359-67. [PubMed] | [CrossRef] | [Google Scholar]
- Singla N, Xie Z, Zhang Z, Gao M, Yousuf Q, Onabolu O, et al. Pancreatic tropism of metastatic renal cell carcinoma. JCI Insight. 2020;5(7):e134564 [PubMed] | [CrossRef] | [Google Scholar]
- Duarte C, Hu J, Beuselinck B, Panian J, Weise N, Dizman N, et al. Metastatic renal cell carcinoma to the pancreas and other sites-a multicenter retrospective study. EClinicalMedicine. 2023;60:102018 [PubMed] | [CrossRef] | [Google Scholar]



