ABSTRACT
Objectives
We aimed to retrospectively evaluate the clinical stage at diagnosis, histopathological features and treatment outcomes of adult patients with malignant melanoma over a 10-year period at Van YüzüncüYıl University Hospital.
Materials and Methods
We reviewed 95 adult patients diagnosed between January 2010 and December 2019. Collected data included demographics, melanoma subtypes, tumor location, AJCC stage, treatment approaches and survival outcomes.
Results
The cohort consisted of 52.6% males and 47.4% females, with a mean age of 56.9±16.4 years. Cutaneous melanoma was the most common subtype (81.1%), with nodular melanoma as the predominant histological type. Lesion location significantly affected survival (*p*<0.05). Stage IV was the most frequent at diagnosis (43.2%). Interferon and chemotherapy were common adjuvant therapies. All ocular melanoma cases underwent surgical enucleation. The median follow-up was 24.4 months. Median overall survival was 11.3 months; the 5-year survival rate was 63.6%.
Conclusion
Our findings emphasize the prognostic impact of histological subtype, tumor site and disease stage at diagnosis. Expanded access to novel therapies may improve outcomes in this patient population.
INTRODUCTION
Malignant melanoma is an aggressive form of skin cancer that originates from the malignant transformation of melanocytes, the pigment-producing cells in the epidermis. Although its incidence is relatively low in Turkey, melanoma contributes substantially to skin cancer-related mortality due to its high metastatic potential and frequent late-stage diagnosis.[1] Early detection is crucial for improving prognosis, as patients diagnosed in primary care settings are more likely to present with early-stage disease and have better outcomes.[2] Prognosis is strongly influenced by clinical stage at diagnosis, anatomical location of the lesion and histopathological features such as Breslow thickness, Clark level, ulceration, mitotic index and growth pattern.[3] These parameters not only reflect tumor biology but also guide treatment strategies.
This study aimed to retrospectively assess the clinical staging, histopathological characteristics and treatment outcomes of patients diagnosed with malignant melanoma at Van YüzüncüYıl University Hospital over a 10-year period. By presenting single-center data from a low-incidence region, this study aims to contribute to the limited body of national data on melanoma in Turkey.
MATERIALS AND METHODS
This retrospective descriptive study included adult patients (≥18 years old) diagnosed with malignant melanoma at Van YüzüncüYıl University Hospital between January 2010 and December 2019. A totalof 95 patientswereidentifiedthroughhospitalmedicalrecords. Histopathological parameters such as Breslow thickness, Clark level, ulceration, mitotic index and growth pattern were evaluated when available. Tumor staging, including histopathological assessment, lymph node involvement and distant metastasis, was performed according to the AJCC T, N and M classifications (Tables 1–3).
| T Classification | Thickness | Ulceration |
|---|---|---|
| Tis | N/A | N/A |
| T1 | ≤1.0 mm | Uncertain or unknown |
| T1a | <0.8 mm | Ulceration absent |
| T1b | <0.8 mm | Ulceration present |
| T1b | 0.8-1 mm | Ulceration present or absent |
| T2 | 1.0-2.0 mm | Uncertain or unknown |
| T2a | 1.0-2.0 mm | Ulceration absent |
| T2b | 1.0-2.0 mm | Ulceration present |
| T3 | 2.0-4.0 mm | Uncertain or unknown |
| T3a | 2.0-4.0 mm | Ulceration absent |
| T3b | 2.0-4.0 mm | Ulceration present |
| T4b | >4.0 mm | Uncertain or unknown |
| T4a | >4.0 mm | Ulceration absent |
| T4b | >4.0 mm | Ulceration present |
| N Classification | Number of Affected Lymph Nodes | Extent of Nodal Involvement |
|---|---|---|
| N0 | No lymph node involvement | |
| N1 | 1 lymph node | |
| N1a | Micrometastasis | |
| N1b | Macrometastasis | |
| N1c | In-transit metastasis/satellites without metastatic nodule | |
| N2 | 2-3 lymph nodes | |
| N2a | Micrometastasis | |
| N2b | Macrometastasis | |
| N2c | In-transit metastasis/satellites without metastatic nodule | |
| N3 | 4 or more lymph nodes | |
| N3a | Micrometastasis | |
| N3b | Macrometastasis | |
| N3c | In-transit metastasis/satellites without metastatic nodule |
| M Classification | Anatomical Site | Serum LDH Level |
|---|---|---|
| M0 | No distant metastasis | |
| M1 | No distant metastasis | |
| M1a | Distant skin, subcutaneous or lymph node metastasis. | |
| M1a(0) | Normal | |
| M1a(1) | Elevated | |
| M1b | Lung metastasis | |
| M1b(0) | Normal | |
| M1b(1) | Elevated | |
| M1c | Visceral metastasis excluding CNS | |
| M1c(0) | Normal | |
| M1c(1) | Elevated | |
| M1d | Central nervous system metastasis | |
| M1d(0) | Normal | |
| M1d(1) | Elevated |
Demographic and clinical data were collected, including age, sex, year of diagnosis, melanoma subtype, tumor location and clinical stage according to the 2017 American Joint Committee on Cancer (AJCC) staging system.[4] The distribution of clinical stages is summarized in Table 4. Treatment strategies and survival outcomes were documented for all patients. Surgical margin assessments were performed in accordance with World Health Organization (WHO) guidelines (Table 5). Treatment protocols administered to each patient subgroup are summarized in Table 6. Statistical analysis was conducted using IBM SPSS Statistics, version 25.0. A p-value <0.05 was considered statistically significant.
| T | N | M | Clinical Staging |
|---|---|---|---|
| Tis | N0 | M0 | 0 |
| T1a | N0 | M0 | IA |
| T1b | N0 | M0 | IB |
| T2a | N0 | M0 | IB |
| T2b | N0 | M0 | IIA |
| T3a | N0 | M0 | IIA |
| T3b | N0 | M0 | IIB |
| T4a | N0 | M0 | IIB |
| T4b | N0 | M0 | IIC |
| Any T, Tis | ≥N1 | M0 | III |
| Any T | Any N | M1 | IV |
| T | N | M | Pathological Staging |
| Tis | N0 | M0 | 0 |
| T1a | N0 | M0 | IA |
| T1b | N0 | M0 | IA |
| T2a | N0 | M0 | IB |
| T2b | N0 | M0 | IIA |
| T3a | N0 | M0 | IIA |
| T3b | N0 | M0 | IIB |
| T4a | N0 | M0 | IIB |
| T4b | N0 | M0 | IIC |
| T0 | N1b, N1c | M0 | IIIB |
| T0 | N2b, N2c, N3b, or N3c | M0 | IIIC |
| T1a/b-T2a | N1a or N2a | M0 | IIIA |
| T1a/b-T2a | N1b/c or N2b | M0 | IIIB |
| T2b/T3a | N1a-N2b | M0 | IIIB |
| T1a-T3a | N2c or N3a/b/c | M0 | IIIC |
| T3b/T4a | Any N ≥N1 | M0 | IIIC |
| T4b | N1a-N2c | M0 | IIIC |
| T4b | N3a/b/c | M0 | IIID |
| Any T, Tis | Any N | M1 | IV |
| Tumor Thickness | Surgical Margin |
|---|---|
| Melanoma in situ | 0.5 cm |
| < 2 mm | 1 cm |
| >2 mm | 2 cm |
| Treatment Protocol | Dose | Administration Schedule |
|---|---|---|
| Interferon alfa-2b | 20 MU/m2 | Days 1-5 |
| (during the first 4 weeks) | ||
| 10MU/m2 | ||
| Days 1, 3 and 5 | ||
| (for 48 weeks) | ||
| Once a week | ||
| Temozolomide | 200 mg/m2 | Days 1-5 |
| Every 28 days | ||
| Paclitaxel | 80 mg/m2 | Weekly |
| Paclitaxel-Carboplatin | 80 mg/m2- AUC=2 | Weekly |
| İpilimumab | 3 mg/kg/gün | Day 1 |
| Every 21 days | ||
| Nivolumab | 1×240 mg | 14 days/Every 21 days |
| Trametenib | 1×2 mg | Continuous |
| Dabrafenib | 2×150 mg | Continuous |
Statistical Analysis
All statistical analyses were performed using IBM SPSS Statistics, version 25.0 (IBM Corp., Armonk, NY, USA). Descriptive statistics were used to summarize the demographic and clinical data. Categorical variables were expressed as frequencies and percentages and continuous variables as mean±standard deviation. Kaplan-Meier survival analysis was used to estimate overall survival and differences between groups were compared using the log-rank test. A *p*-value <0.05 was considered statistically significant.
RESULTS
Patient Characteristics
This study included 95 patients, comprising 50 males (52.6%) and 45 females (47.4%), with a mean age of 56.9±16.4 years (range: 24-90). Detailed demographic characteristics are presented in Table 7.
| Characteristics | Number of Patients | Percentage (%) | |
|---|---|---|---|
| Sex | Male | 50 | 52,6 |
| Sex | Female | 45 | 47,4 |
| Melanoma Type | Skin | 73 | 76,8 |
| Melanoma Type | Mucosal | 8 | 8,4 |
| Melanoma Type | Ocular | 9 | 9,4 |
| Melanoma Type | Unknown | 5 | 5,2 |
| Localization | Ocular | 9 | 9,5 |
| Localization | Skin | 11 | 11,6 |
| Localization | Head and Neck | 21 | 22,1 |
| Localization | Trunk | 9 | 9,5 |
| Localization | Lower Extremity | 9 | 9,5 |
| Localization | Upper Extremity | 23 | 24,2 |
| Localization | Mucosal | 5 | 5,3 |
| Localization | Oral Mucosa | 1 | 1,1 |
| Localization | Rectum | 2 | 2,1 |
| Localization | Unknown | 5 | 5,3 |
| Type of Surgery | Tumor Excision | 58 | 86,6 |
| Type of Surgery | Enucleation | 9 | 13,4 |
| Lymph Node Involvement | N0 | 47 | 78,3 |
| Lymph Node Involvement | N1 | 2 | 3,3 |
| Lymph Node Involvement | N2 | 2 | 3,3 |
| Lymph Node Involvement | N3 | 9 | 15 |
| Metastasis | Absent | 50 | 79,4 |
| Metastasis | Present | 13 | 20,6 |
| At Diagnosis Metastasis | Liver | 12 | 34,2 |
| At Diagnosis Metastasis | Brain | 5 | 14,2 |
| At Diagnosis Metastasis | Skin | 2 | 5,7 |
| At Diagnosis Metastasis | Lung | 10 | 28,5 |
| At Diagnosis Metastasis | Abdomen | 6 | 17,1 |
| During Follow-up Metastasis | Liver | 4 | 12,9 |
| During Follow-up Metastasis | Brain | 7 | 22,6 |
| During Follow-up Metastasis | Abdomen | 14 | 45,2 |
| During Follow-up Metastasis | Lung | 6 | 19,4 |
| MaleCOG | 0 | 40 | 42,1 |
| 1 | 36 | 37,9 | |
| 2 | 15 | 15,8 | |
| 3 | 4 | 4,2 | |
| Final Status | Malex | 34 | 35,8 |
| Alive | 61 | 64,2 |
Melanoma Subtypes and Localization Cutaneous melanoma was the most common subtype (81.1%, n=73), followed by ocular (10.0%, n=9) and mucosal melanoma (8.9%, n=8). In five patients, the primary tumor site could not be identified. Nodular melanoma was the most prevalent histological subtype (46.7%, n=28), followed by lentigo maligna (21.7%, n=13), superficial spreading melanoma (13.3%, n=8) and acral lentiginous melanoma (13.3%, n=8). Information on histological subtype was missing in 35 cases. The extremities were the most frequent tumor location (33.7%, n=32), with the lower limbs accounting for 71.8% of these cases, followed by the head and neck region (22.1%) and the trunk (9.5%).
Histopathological Findings
Breslow thickness was available in 43 patients: 24.5% had tumors
≤1 mm, 18.9% were 1.01-2.00 mm, 11.3% were 2.01-4.00 mm and 45.3% were >4 mm. Clark level was documented in 42 patients, with level IV being most common (54.7%). Ulceration was present in 39.7% of evaluable cases (n=27/68). Vertical growth was observed in 78.6% of cases (n=48/61). The mean mitotic index was 13.39±17.12. Pathological features are summarized in Table 8. Staging and Survival Outcomes Based on AJCC 2017 criteria, 43.2% of cutaneous melanoma patients were diagnosed at stage IV, 32.8% at stage II, 19.4% at stage I and 4.4% at stage III. The median follow-up period was 24.4 months (range: 0.1-222.8). During follow-up, 35.7% (n=34) of patients died. The median overall survival among deceased patients was 11.3 months, with a mean survival of 20.6 months. The Kaplan-Meier curve for overall survival is shown in Figure 1. The 5-year survival rate was 63.6%.
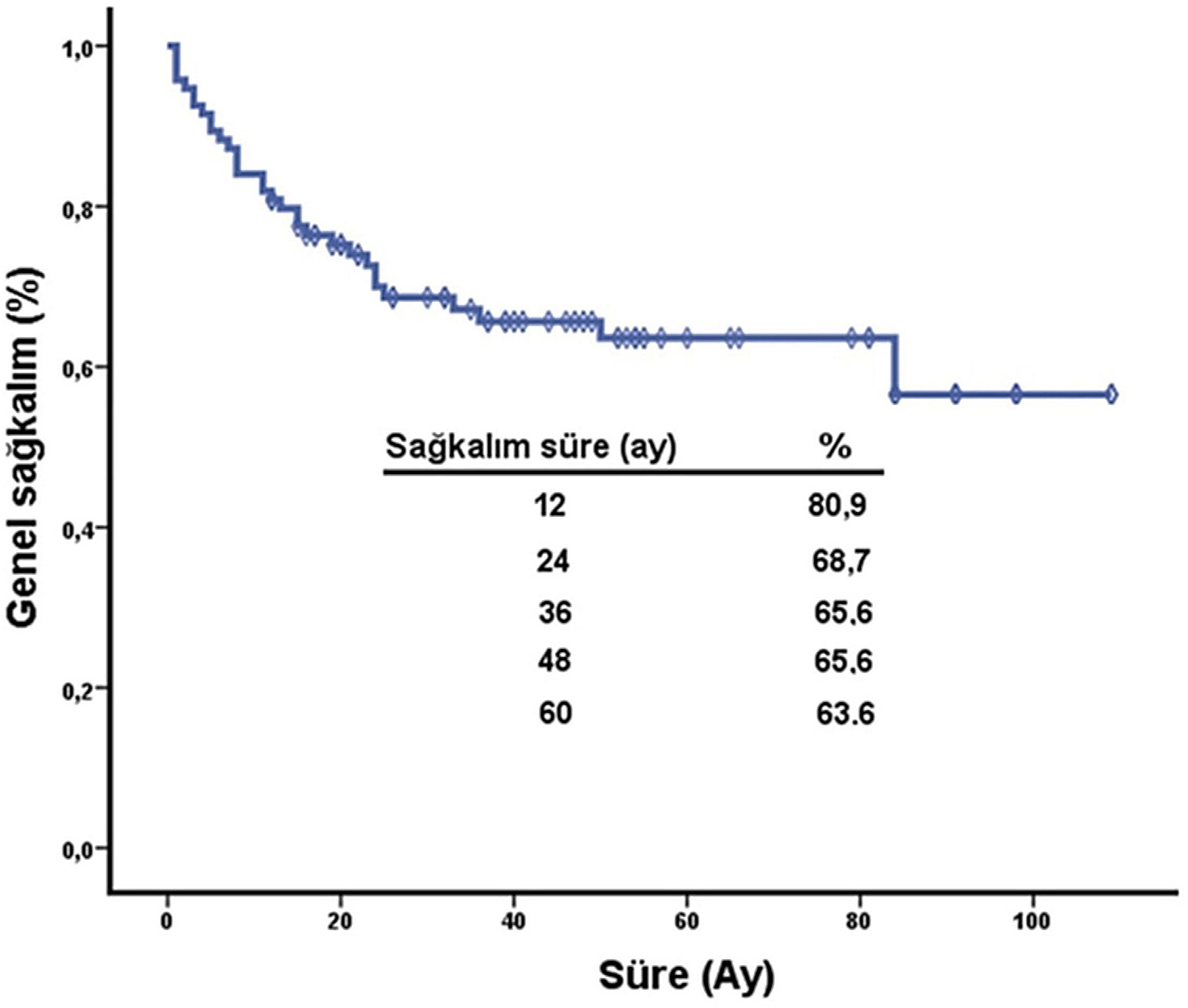
Figure 1:
Kaplan-Meier overall survival curve for all patients with malignant melanoma (n=95). The median overall survival was 11.3 months (95% CI: 8.5-14.2). Censored data are indicated with vertical ticks.
| Characteristics | Number of Patients | Percentage (%) | |
|---|---|---|---|
| Histology | Acral Lentiginous | 8 | 13,3 |
| Histology | Nodular | 28 | 46,7 |
| Histology | Lentigo Maligna | 13 | 21,7 |
| Histology | Superficial spreading | 8 | 13,3 |
| Histology | Ocular | 3 | 5 |
| Growth Phase | Absent | 34 | 35,8 |
| Growth Phase | Radial | 13 | 13,7 |
| Growth Phase | Vertical | 48 | 50,5 |
| Satellite Nodule | Absent | 31 | 73,8 |
| Satellite Nodule | Present | 11 | 26,2 |
| Ulceration | Absent | 41 | 60,3 |
| Ulceration | Present | 27 | 39,7 |
| LVI (Lymphovascular Invasion) | Absent | 38 | 73,1 |
| LVI (Lymphovascular Invasion) | Present | 14 | 26,9 |
| PNI (Perineural Invasion) | Absent | 33 | 84,6 |
| PNI (Perineural Invasion) | Present | 6 | 15,4 |
| Lymphocytic Infiltration | Absent | 52 | 57,1 |
| Lymphocytic Infiltration | Present | 39 | 42,9 |
| Surgical Margin | Absent | 45 | 83,3 |
| Surgical Margin | Present | 9 | 16,7 |
| Breslow Thickness (mm) | ≤1 mm | 13 | 24,5 |
| Breslow Thickness (mm) | 1.01-2.00 mm | 10 | 18,9 |
| Breslow Thickness (mm) | 2.01-4.00 mm | 6 | 11,3 |
| Breslow Thickness (mm) | > 4 mm | 24 | 45,3 |
| Clark Level | Unspecified | 43 | 50,6 |
| Clark Level | Level 1 | 6 | 7,1 |
| Clark Level | Level 2 | 4 | 4,7 |
| Clark Level | Level 3 | 9 | 10,6 |
| Clark Level | Level 4 | 23 | 27,1 |
| BRAF Mutation | Absent | 14 | 77,8 |
| BRAF Mutation | Present | 4 | 22,2 |
Prognostic Factors
Survival was significantly associated with melanoma subtype (*p*<0.001). Median survival was 24 months for cutaneous melanoma, 11 months for mucosal melanoma and not reached for ocular melanoma.[5] The distribution of clinical stages at initial diagnosis is illustrated in Figure 2. Among cutaneous subtypes, mean survival was 27.1 months (acral lentiginous), 26.7 months (lentigo maligna), 24.4 months (superficial spreading) and 13.5 months (nodular). Lesion location (*p*<0.05), lymph node involvement (median survival 24.4 months vs. 6.27 months) and distant metastasis (*p*<0.05) were also significant prognostic factors.
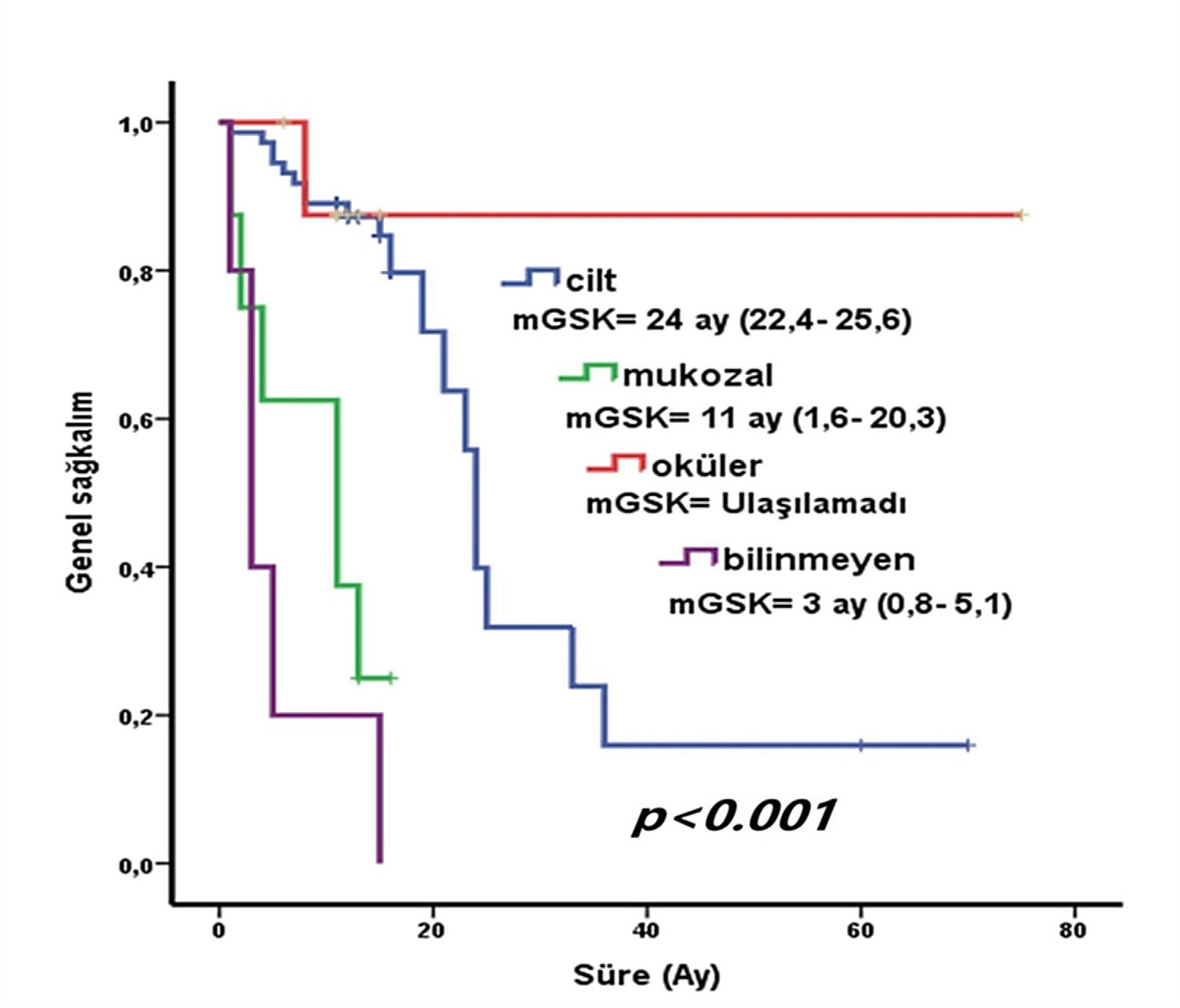
Figure 2:
Distribution of patients by AJCC clinical stage at initial diagnosis. Stage IV was the most frequently observed (43.2%), followed by Stage III (25.3%). The percentage of patients in each stage is shown.
Treatment
Five patients received adjuvant interferon therapy. Among 17 patients with metastatic cutaneous melanoma, 11 were treated with temozolomide, 4 with nivolumab, 4 with ipilimumab and 2 with vemurafenib. All ocular melanoma cases underwent surgical enucleation. Treatment strategies are summarized in Table 9.
| Treatment Type / Line | Number of Patients | Percentage (%) | |
|---|---|---|---|
| Surgical | Tumor Excision | 57 | 60 |
| Surgical | Enucleation | 9 | 9,4 |
| Radiotherapy | Adjuvant | 2 | 2,1 |
| Radiotherapy | Palliative | 2 | 2,1 |
| Adjuvant interferon therapy | 5 | 5,2 | |
| First-line palliative chemotherapy | Temozolomide | 9 | 56,2 |
| First-line palliative chemotherapy | Temozolomide+Cis | 2 | 12,5 |
| First-line palliative chemotherapy | Trametinib | 1 | 6,25 |
| First-line palliative chemotherapy | Dabrafenib+Trametinib | 1 | 6,25 |
| First-line palliative chemotherapy | Nivolumab | 1 | 6,25 |
| First-line palliative chemotherapy | Paclitaxel-Carboplatin | 1 | 6,25 |
| First-line palliative chemotherapy | Thalidomide | 1 | 6,25 |
| Second-line palliative chemotherapy | Temozolomide | 1 | 12,5 |
| Second-line palliative chemotherapy | Ipilimumab | 2 | 25 |
| Second-line palliative chemotherapy | Vemurafenib | 2 | 25 |
| Second-line palliative chemotherapy | Paclitaxel | 1 | 12,5 |
| Second-line palliative chemotherapy | Nivolumab | 2 | 25 |
| Third-line palliative chemotherapy | Ipilimumab | 2 | 50 |
| Third-line palliative chemotherapy | Nivolumab | 1 | 25 |
| Third-line palliative chemotherapy | Temozolomide | 1 | 25 |
DISCUSSION
A notable limitation of our study was the incomplete documentation of histopathological data, such as missing values for Breslow thickness and histological subtype in a significant proportion of patients. Additionally, as a retrospective study conducted at a single tertiary center, selection and information bias may have influenced the results. Lack of access to novel therapies and variability in treatment protocols over the 10-year study period may also limit the generalizability of our findings. Recent clinical trials have demonstrated improved survival with immunotherapy and targeted therapies such as nivolumab, ipilimumab, and vemurafenib in advanced melanoma cases. [6–10]
CONCLUSION
This study provides a comprehensive overview of malignant melanoma cases diagnosed over a decade at a tertiary center in Eastern Turkey. Nodular melanoma emerged as the most prevalent histological subtype, with a substantial proportion of patients presenting at advanced clinical stages. Tumor localization and disease stage were identified as key prognostic factors influencing survival outcomes.
These findings underscore the critical importance of early detection, standardized histopathological evaluation and equitable access to contemporary systemic therapies. Public health strategies aimed at enhancing awareness and facilitating timely dermatological consultations, along with national policies to improve the availability of targeted and immunotherapeutic agents, are essential for optimizing outcomes in similarly low-incidence regions.
Cite this article:
Turkoglu Z, Esen R. Clinical Staging, Histopathological Features and Treatment Outcomes in Malignant Melanoma: A 10-Year Retrospective Single-Center Study. Journal of BUON. 2025;28:x-x.
ACKNOWLEDGEMENT
The authors thank the medical records and pathology departments of Van Yüzüncü Yıl University Hospital for their support in data retrieval and verification.
The authors thank the medical records and pathology departments of Van Yüzüncü Yıl University Hospital for their support in data retrieval and verification.
ABBREVIATIONS
| AJCC | American Joint Committee on Cancer |
|---|---|
| WHO | World Health Organization |
| mOS | Median Overall Survival |
| CI | Confidence Interval |
| MM | Malignant Melanoma. |
References
- Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics. CA Cancer J Clin. 2023;73(1):17-48. [CrossRef] | [Google Scholar]
- Watts CG, Madronio CM, Morton RL, Goumas C, Inskip M, Armstrong BK, et al. Diagnosis and survival experience of Australians with melanoma treated in primary, secondary, or tertiary settings. Br J Dermatol. 2017;176(1):135-41. [CrossRef] | [Google Scholar]
- Swetter SM, Tsao H, Bichakjian CK, Curiel-Lewandrowski C, Elder DE, Gershenwald JE, et al. Guidelines of care for the management of primary cutaneous melanoma. J Am Acad Dermatol. 2019;80(1):208-50. [CrossRef] | [Google Scholar]
- Balch CM, Gershenwald JE, Soong SJ, Thompson JF, Atkins MB, Byrd DR, et al. Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol. 2009;27(36):6199-206. [CrossRef] | [Google Scholar]
- Tas F, Erturk K. Clinicopathological characteristics and prognosis of cutaneous and noncutaneous melanoma: a single-center experience. Med Oncol. 2017;34(6):105 [CrossRef] | [Google Scholar]
- Robert C, Ribas A, Hamid O, Daud A, Wolchok JD, Hodi FS, et al. Durable complete response after discontinuation of immunotherapy in patients with metastatic melanoma. J Clin Oncol. 2018;36(17):1668-74. [CrossRef] | [Google Scholar]
- Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Cowey CL, Lao CD, et al. Five-year survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med. 2019;381(16):1535-46. [CrossRef] | [Google Scholar]
- Ascierto PA, Del Vecchio M, Mackiewicz A, Robert C, Chiarion-Sileni V, Arance A, et al. Overall survival at 5 years of follow-up in a phase III study of vemurafenib vs. dacarbazine in BRAF-mutated metastatic melanoma. Eur J Cancer. 2020;132:99-106. [CrossRef] | [Google Scholar]
- Luke JJ, Flaherty KT, Ribas A, Long GV. Targeted agents and immunotherapies: optimizing outcomes in melanoma. Nat Rev Clin Oncol. 2017;14(8):463-82. [CrossRef] | [Google Scholar]
- Garbe C, Peris K, Hauschild A, Saiag P, Middleton M, Bastholt L, et al. Diagnosis and treatment of melanoma. European consensus-based interdisciplinary guideline-Update 2022. Eur J Cancer. 2023;178:86-103. [CrossRef] | [Google Scholar]



