ABSTRACT
Abdominal aortic aneurism (AAA) is a frequent disorder in older male adults; male to female ratio is approximately 10:3. AAA often causes significant morbidity and mortality if not resolved on time by early detection and timely performed endovascular or open surgical repair. The incidence of rupture with an AAA <7 cm diameter is < 5% per 1 year. This gives enough time to many patients for improvement of possible pre-existing medical conditions before elective repair. There is no evidence that radiation therapy causes aortic aneurysm. In contrast, there is a possibility that radiation slows the growth of AAA. The mechanism of this action is not known.
INTRODUCTION
Prolonged life expectancy leads in elderly patients to multimorbidity.[1] For example, Abdominal aortic aneurism (AAA) occurs concomitantly with malignancy in 1.0 to 17.0% of patients.[2] Radiation therapy (RT) plays a prominent role in the treatment of many cancers since tumors and healthy tissues show significant differences in sensitivity to radiation. That is the reason why diseased tissue faster responds to radiation than healthy tissue, and clinical manifestations of side effects caused by RT may occur years later.[3,4]
The aim of this article is to present abdominal aortic aneurysm, and to show impact of radiation on this vascular defect.
Abdominal aortic aneurysm
The expansion of artery lumen by 50% in relation to the adjacent part of the vessel presents an aneurysm.[5] When infrarenal dilatation of the aorta is in question, it is an AAA. Such change is in most cases without symptoms, until the aneurysm progresses to rupture. The greatest risk for the occurrence of aneurysm occurs in men at the beginning of geriatric age (65 years); the ratio male to female is approximately 10:3. Thoracic aortic aneurysm exhibits no gender difference and it is more prevalent in younger individuals.[6]
Because there are no effective medical therapies, current clinical intervention for large aneurysm includes two options: Endovascular repair (EVAR) and open surgical repair. EVAR is used for both elective repair and at a ruptured case; a bifurcated or tubular stent-graft over the AAA excludes the aneurysm from arterial circulation. This procedure is superior to open surgical repair. Type II endoleak, with an occurrence rate of 20-30%, is the most common complication after EVAR.[7] Elective EVAR results in lower perioperative mortality than traditional open repair, but after 4 years the EVAR survival advantage is not seen; the results of two European trials have also shown worse long-term outcomes with EVAR than with open repair.[8]
A time for the repair has been recommended when the infrarenal aorta diameter becomes larger than 5.5 cm in men and 5.0 cm in women, due to high incidence of ruptures in non-operated aneurysms.[9] This opinion is based on the data obtained 2002 which show a 9% risk of rupture for aneurysms of 5.5-5.9 cm in diameter within 1 year, compared with 19% for those measuring 6.0-7.0 cm, and 33% for > 7.0 cm in diameter. However, recent studies have questioned this because the incidence of rupture in patients with an AAA <7.0 cm diameter was < 5% per 1 year.[10] This finding gives enough time to many patients for improvement of the pre-existing medical conditions before surgical intervention. With AAA diameter above 7.0 cm, a patient faces much higher risk of rupture. The U.S. Preventive Services Task Force recommends that men with a history of smoking who are 65 to 75 years of age should undergo one-time abdominal aortic aneurysm screening with ultrasonography.
Proper control of hypertension, tobacco smoking, diabetes, and blood lipids may partially slow down AAA growth. However, ACE inhibitors should not be used for blood pressure control in such patients. It is better to use one of the ARB (e.g. losartan, irbesartan, valsartan), because ACE also acts as a kininase. In fact, ACE and kininase II (Figure 1) are the same enzyme.[11,12] Therefore, administration of an ACE inhibitor has a dual action, it decreases angiotensin II and increases bradykinin levels. The latter peptide may stimulate aneurysm’s expansion.
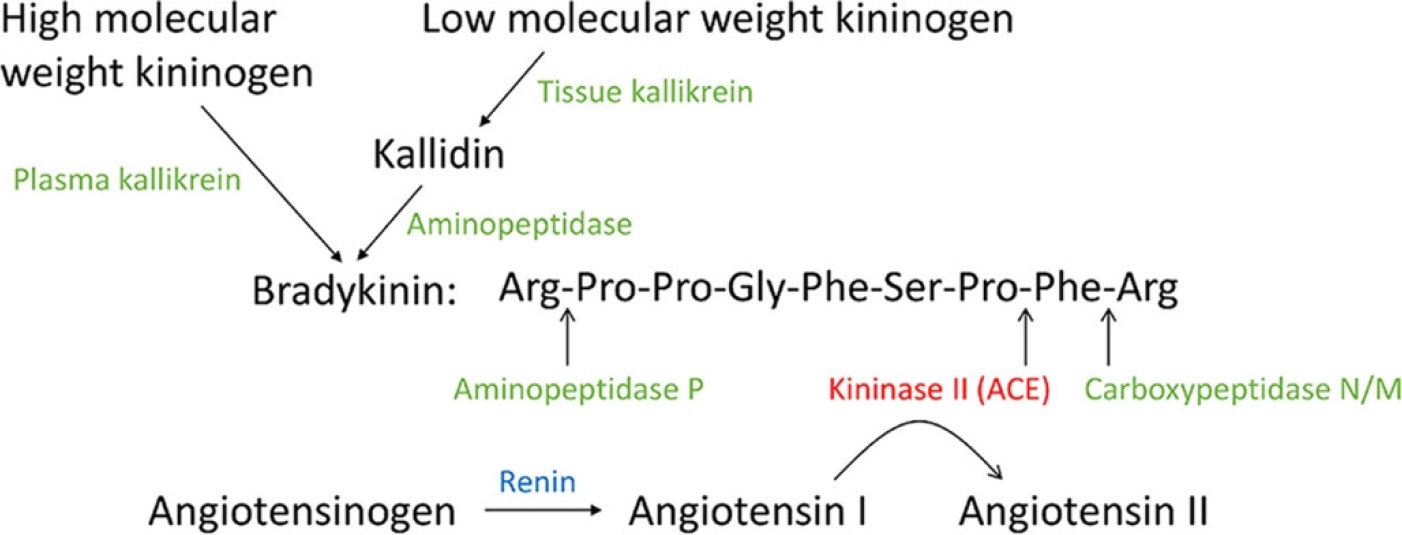
Figure 1:
The peptides and peptidases in the Kallikrein-Kinin System (KKS) and Renin Angiotensin System (RAS). The enzymes in the KKS are shown in green, and renin is shown in blue; ACE (kininase II) is shown in red.
The loss of vascular smooth muscle cells and degradation of the extracellular matrix leads to formation of AAA. New experimental approaches for medical treatment are promising; mouse aneurysm models are frequently used-such as angiotensin II induced abdominal aortic aneurysm in male apolipoprotein E knockout mice (Apoe-/-).[13,14] Pentamethyl quercetin, which inhibits angiotensin II-induced abdominal aortic aneurysm formation by binding to C/EBPβ at Lys253, and reduces the incidence of AAA rupture in mice.[15] Asiatic acid, a triterpene compound, has also been investigated due to its strong anti-inflammatory property. This compound induces aortic remodeling and is useful against blood vessel dissection in the murine model.[16]
Psychic changes in AAA patients
Cases of sudden death due to aneurysm rupture were rarely described in the distant past. An example was described in 1887; Alexander Borodin, a professor of pathology and famous Russian composer died at age of 55 due to the rupture of coronary artery aneurysm.[17] Coronary ar.tery aneurysm can cause death due to thrombosis or rupture. It is usually associated with destruction of the tunica media, due to atherosclerosis and inflammation.[18] RT my cause atherosclerosis, including coronary artery disease, valvular disease, constrictive pericarditis and heart failure.[19] Today, abdominal and thoracal scanning examinations have revealed the presence of the AAA in many persons and this expanded public knowledge of what danger the aneurysm presents. When an aneurysm is discovered in a patient, especially the AAA, this may cause psychological disorders, including depression that occurs more often in older subjects. That is why it is necessary to monitor the psychiatric status of a patient with aneurysm and perform emergency intervention when necessary.[20] Additional problem presents significantly increased (33%) incidence of delirium following abdominal aortic repair.[21]
Radiation therapy and AAA
In addition to cancerous cells, ionizing radiation also affects rapidly proliferating cells; e.g. endothelial and bone marrow cells. DNA damage causes the cell cycle arrest and apoptosis of healthy tissue. High doses result in depletion of vascular endothelial cells and both macro- and microvascular effects are induced.[22] The radiation therapy is associated with an increased risk for cardiovascular damage; for example, neck and cranial RT have been associated with significant long-term toxicities including accelerated occlusive carotid artery disease, autonomic dysfunction due to baroreceptor harm, and development of metabolic syndrome caused by damage to the hypothalamic-pituitary axis.[23] In addition, intracranial aneurysms often appear after radiation therapy.[24,25] Modern radiotherapy techniques reduce the damage of heart and major coronary vessels exposed to high doses, yet some exposure is frequently unavoidable and some radiation damage occurs. For example, inflammatory changes in the microvasculature cause myocardial damage, local ischemia, progressive myocardial cell death and fibrosis. Irradiation of large vessels causes endothelial cell lining damage and this leads to adhesion of circulating monocytes who transform into activated macrophages and the process of atherosclerosis is initiated, microthrombi are formed and vessels occluded.[26] Clinical studies also demonstrate regional perfusion defects in non-symptomatic breast cancer patients after radiotherapy.
Therefore, it is unexpected finding that radiation therapy for pelvic cancers, including prostate carcinoma, reduces abdominal aneurysm growth.[27,28] This finding would be worth examining in more detail and determine the effect of the RT at the cellular and molecular level.
ACE activity in rat aorta was increased 1-24 hr after whole body irradiation in a dose of 2.5 Gy with a peak in 2 hr after exposure.[29] Male Wistar rats were exposed to whole body or local (chest) X-ray irradiation (200 kV, 1-7.5 Gy). Such radiation dose is equal to one fraction dose that is used in tumor radiation. The activity of the enzyme in aorta segments was measured in 1-48 hr after irradiation by hydrolysis of hippuryl-histidine-leucine substrate. The early effect of radiation is probably caused by the influence of radiation at the aortic endothelial cells. However, the long-term changes due to increased enzyme activity on the AAA is not determined.
LIMITATION
This paper has not focused on the RT risk at the separate occurrence of the AAA in men and women.
CONCLUSION
It is well known that ionizing radiation affects both cancerous and non-cancerous cells, especially rapidly proliferating cells, e.g. endothelial cells. There is no evidence that radiation therapy causes abdominal aortic aneurysm. On the contrary, there are data that radiotherapy slows the growth of abdominal aorta aneurysm. The mechanism of this action is unknown.
References
- Souza DLB, Oliveras-Fabregas A, Minobes-Molina E, de Camargo Cancela M, Galbany-Estragues P, Jerez-Roig J, et al. Trends of multimorbidity in 15 European countries: a population-based study in community-dwelling adults aged 50 and over. BMC Public Health. 2021;21:76 [CrossRef] | [Google Scholar]
- Maxwell DW, Kenney L, Sarmiento JM, Rajani RR. Aortic aneurysm natural progression is not influenced by concomitant malignancy and chemotherapy. Annals of Vascular Surgery, 2021. 2021;71:29-39. [CrossRef] | [Google Scholar]
- Armanious MA, Mohammadi H, Khodor S, Oliver DE, Johnstone PA, Fradley MG, et al. Cardiovascular effects of radiation therapy. Current Problems of Cancer. 2018;42:433-442. [CrossRef] | [Google Scholar]
- Mileusnić D, Maroševic G, Durbaba M. Radijaciona onkologija [Radiation oncology]. 2020 [CrossRef] | [Google Scholar]
- Lindeman JH, Matsumura JS. Pharmacologic management of aneurysms. Circulation Research. 2019;124:631-646. [CrossRef] | [Google Scholar]
- Guo DC, Papke CL, He R, Milewicz DM. Pathogenesis of thoracic and abdominal aortic aneurysms. Annals of the New York Academy of Science. 2006;1085:339-352. [CrossRef] | [Google Scholar]
- Akmal MM, Pabittei DR, Prapassaro T, Suhartono R, Moll FL, van Herwaarden JA, et al. A systematic review of the current status of interventions for type II endoleak after EVAR for abdominal aortic aneurysms. International Journal of Surgery. 2021;95:106-138. [CrossRef] | [Google Scholar]
- Lederle FA, Kyriakides TC, Stroupe KT, Freischlag JA, Padberg FT, Matsumura JS, Huo Z, Johnson GR, et al. OVER Veterans Affairs Cooperative Study Group. Open versus endovascular repair of abdominal aortic aneurysm. New England Journal of Medicine. 2019;380(22):2126-2135. [CrossRef] | [Google Scholar]
- Lederle FA, Johnson GR, Wilson SE, Ballard DJ, Jordan WD, Blebea J, et al. Rupture rate of large abdominal aortic aneurysms in patients refusing or unfit for elective repair. JAMA. 2002;287 [CrossRef] | [Google Scholar]
- Scott SWM, Batchelder AJ, Kirkbride DS, Naylor AR, Thompson JP. Late survival in nonoperated patients with infrarenal abdominal aortic aneurysm. European Journal of Vascular and Endovasular Surgery. 2016;52:444-449. [CrossRef] | [Google Scholar]
- Igić R, Behnia R. Properties and distribution of angiotensin I converting enzyme. Current Pharmaceutical Design. 2003;9:697-706. [CrossRef] | [Google Scholar]
- Igić R. An exploration of bioactive peptides: My collaboration with Ervin G. Erdös. Journal of Biological Chemistry. 2018;293:7907-7915. [CrossRef] | [Google Scholar]
- Thirunavukkarasu S, Khan NS, Song CY, Ghafoor HU, Brand DD, Gonzalez FJ, Malik KU, et al. Cytochrome P450 1B1 contributes to the development of angiotensin II-induced aortic aneurysm in male Apoe (-/-) mice. American Journal of Pathology. 2016;186:2204-2219. [CrossRef] | [Google Scholar]
- Mukherjee K, Pingili AK, Singh P, Dhodi AN, Dutta SR, Gonzalez FJ, Malik KU, et al. Testosterone metabolite 6β-hydroxytestosterone contributes to angiotensin II-induced abdominal aortic aneurysms in Apoe-/- male mice. Journal of the American Heart Association. 2021;10(7) [CrossRef] | [Google Scholar]
- Wu H, Wang J, Bu Y, Li J, Li Y, Jing Q, Wang X, Yan C, Liu D, Han Y, et al. Pentamethyl quercetin attenuates angiotensin II-induced abdominal aortic aneurysm formation by blocking nuclear translocation of C/EBPβ at Lys253. Biochimca et Biophysica Acta – Molecular Basis of Disease. 2024;1870(5) [CrossRef] | [Google Scholar]
- Zhang H, Li Y, Liu M, Guo M, Zhang R, Zhao K, Wu J, Zhao Z, Zhu H, Liu J, et al. Asiatic acid alleviates vascular remodeling in BAPN-induced aortic dissection through inhibiting NF-κB p65/CX3CL1 signaling. FASEB Journal. 2024;38(9) [CrossRef] | [Google Scholar]
- Igić R. Alexander Borodin’s contributions to arts and sciences. Scripta Medica. 2023;54:301-305. [CrossRef] | [Google Scholar]
- Nichols L, Lagana S, Parwani A. Coronary artery aneurysm: a review and hypothesis regarding etiology. Archives of Pathology and Laboratory Medicine. 2008;132(5):823-8. [CrossRef] | [Google Scholar]
- Mitchell JD, Cehic DA, Morgia M, Bergom C, Toohey J, Guerrero PA, Ferencik M, Kikuchi R, Carver JR, Zaha VG, Alvarez-Cardona JA, Szmit S, Daniele AJ, Lopez-Mattei J, Zhang L, Herrmann J, Nohria A, Lenihan DJ, Dent SF, et al. Cardiovascular manifestations from therapeutic radiation: A multidisciplinary expert consensus statement from the International Cardio-Oncology Society. JACC: CardioOncology. 2021;3(3):360-380. [CrossRef] | [Google Scholar]
- Kim MH, Yoo JH, Cho HJ, Ko KJ, Jun KW, Han KD, Hwang JK, et al. Increased depression risk in patients with abdominal aortic aneurysm: a nationwide cohort study. Annals of Surgical Treatment and Research. 2021;101:291-298. [CrossRef] | [Google Scholar]
- Besselink-Lobanova A, Van der Meer NJ, Van der Laan L. Abdominal aortic aneurysm patients remain at risk for delirium on the surgical ward after intensive care unit dismissal. Minerva Anestesiologica. 2020;86:930-938. [CrossRef] | [Google Scholar]
- Yang EH, Marmagkiolis K, Balanescu DV, Hakeem A, Donisan T, Finch W, Virmani R, Herrman J, Cilingiroglu M, Grines CL, Toutouzas K, Iliescu C, et al. Radiation-induced vascular disease-a state-of-the-art review. Frontiers in Cardiovascular Medicine. 2021;8 [CrossRef] | [Google Scholar]
- Texakalidis P, Giannopoulos S, Tsouknidas I, Song S, Rivet DJ, Reiter ER, Reavey-Cantwell J, et al. Prevalence of carotid stenosis following radiotherapy for head and neck cancer: a systematic review and meta-analysis. Head and Neck. 2020;42:1077-1088. [CrossRef] | [Google Scholar]
- Casey AT, Marsh HT, Uttley D. Intracranial aneurysm formation following radiotherapy. British Journal of Neurosurgery. 1993;7:575-579. [CrossRef] | [Google Scholar]
- Jensen FK, Wagner A. Intracranial aneurysm following radiation therapy for medulloblastoma. A case report and review of the literature. Acta Radiologica. 1997;38:37-42. [CrossRef] | [Google Scholar]
- Stewart FA, Hoving S, Russell NS. Vascular damage as an underlying mechanism of cardiac and cerebral toxicity in irradiated cancer patients. Radiation Research. 2010;174(6):865-9. [CrossRef] | [Google Scholar]
- Becker von Rose A, Kobus K, Bohmann B, Lindquist-Lilljequist M, Eilenberg W, Bassermann F, Reeps C, Eckstein HH, Trenner M, Maegdefessel L, Neumayer C, Brostjan C, Roy J, Hultgren R, Schwaiger BJ, Busch A, et al. Radiation and chemotherapy are associated with altered aortic aneurysm growth in patients with cancer: Impact of synchronous cancer and aortic aneurysm. European Journal of Vascular and Endovascular Surgery. 2022;64:255-264. [CrossRef] | [Google Scholar]
- Becker von Rose A, Kobus K, Bohmann B, Lindquist-Lilljequist M, Eilenberg W, Kapalla M, Bassermann F, Reeps C, Eckstein HH, Neumayer C, Brostjan C, Roy J, von Heckel K, Hultgren R, Schwaiger BJ, Combs SE, Busch A, Schiller K, et al. Radiation therapy for cancer is potentially associated with reduced growth of concomitant abdominal aortic aneurysm. Strahlentherapie und Onkologie. 2024;200:425-433. [CrossRef] | [Google Scholar]
- Korystova AF, Kublik LN, Levitman MK, Samokhvalova TV, Shaposhnikova VV, Korystov YN, et al. Ionizing radiation enhances activity of angiotensin converting enzyme in rat aorta. Bulletin of Experimental Biology and Medicine. 2018;165:216-219. [CrossRef] | [Google Scholar]



