ABSTRACT
Melanoma is a type of skin cancer which originates from melanocytes, the cells that produce the pigment melanin. Melanoma is one of the most aggressive and lethal forms of cancer, as it can rapidly spread to other organs if not detected and treated early. The history of melanoma can be traced back to antiquity, named after the Greek term “melas” which mean black. The history of treatment of melanoma reflects the advances in understanding the biology, pathology, and immunology of this disease, as well as the development of novel therapeutic strategies that target specific molecular pathways or immune responses. Important figures like John Hunter, Wallace Clark and Alexander Breslow are being presented, alongside their pioneering work. Herein, this narrative review is about the most significant medical landmarks in the history of melanoma over the past 3 centuries.
INTRODUCTION
Melanomas were initially recognized as firm, dark blood clot like entities that had been organized at their center. Even though this was a malignancy, its beginning presented no signs, but only a concentration of a pigment in the deeper layers of the cutis vera. Their progression was characterized as sarcomatous or carcinomatous with no rapid dissemination.[1] Among the earliest references was that of Thomas Fawdington (1795-1843), who stated in 1826 for a case of ocular melanoma “As we have now positive indications to guide towards the cure of melanosis, the treatment directed is of course, purely palliative.[2] The term exists in the Hellenic Lexicon of Skarlatos Byzantios (1797/1798-1878) of 1874.[3] Metastatic melanoma is one of the most challenging cancers to be treated even under nowadays anticancer protocols. Melanoma is 20 times more common among the white race than the black. The risk of melanoma for white people is about 2.6% in a lifetime in comparison with only 0.1% for black people. Melanoma incidence has been increasing steadily in the past decades, especially in white-skinned populations. This is mainly due to the increased exposure to Ultraviolet (UV) radiation from the sun or artificial sources, such as tanning beds. Other factors that may influence melanoma incidence include genetic susceptibility, skin type, age, sex, and geographic location.[4] Historically, melanomas are subdivided into superficial spreading, nodular, lentigo maligna and acral lentiginous melanoma with more subtypes to have subsequently been added, while quite a few variants exist, such as desmoplastic, nevoid, malignant blue nevus and pigment synthesizing melanoma.[5]
Melanoma incidence in antiquity is difficult to be estimated, as no reliable records or data exist. However, some evidence suggests that melanoma was a rare condition in ancient civilizations, as it was only mentioned sporadically by ancient physicians and historians. Some possible reasons for the low incidence of melanoma in ancient times are: i) people had darker skin types that were more resistant to UV damage, ii) people wore more clothing and hats that covered their skin from the sun, iii) people spent less time outdoors or in high-altitude areas where UV radiation is stronger and iv) people had shorter life spans and died of other causes before developing a melanoma. The history of melanoma is vast, extending well into antiquity, with the first credible literary description mention documented by Hippocrates in the 5th century BC, followed some eons later by the Greek physician Rufus of Ephesus.[6–7] The term melanoma comes from the ancient Greek language, when “melano” meant black and to second part of the word “oma” signified a tumor.[8]
This narrative review aims to present the historical pearls of a fatal entity known since antiquity. For an oncologist to be acquainted with the history of a disease he cures, is essential for the comprehension of its profession itself.
Defining the Origins
The earliest testimony of melanoma comes from the diffuse melanotic metastasis found in the skeletons of pre-Columbian mummies of Incas radiocarbon dated to be ca 2400 years old from Chancay and Chingas in Peru. Paradoxically in 18th century, the black moles in women were admired in France as a part of beauty, as further described by poet Richardson Pack in one of his odes “Dear Molly Spring”. Examples exist of painters depicting realistic images of melanotic masses in their work (Figure 1). In another aspect, moles had religious significance. The 14th Dalai Lama should have had prominent ears and moles on the upper trunk according to the book “7 years in Tibet”.[9] The first melanoma specimen had been excised by the Scottish surgeon John Hunter (1728-1793) in 1787, which came from a 35 years old male, a recurrent mass from behind the angle of the mandible. This specimen was described by Hunter as a “cancerous fungous excrescence”. In 1804, French physician René-Théophile-Hyacinthe Laennec (1781-1826) first described melanoma as a distinct disease in Paris and coined the term melanosis (la melanosa) as published in 1812 inside the “Bulletin de la Faculté de Médecine de Paris”. Laennec suggested that four forms of melanoses existed, a disease infiltrating into an organ, or disseminating various surface deposits on an organ. Military French surgeon and famous anatomist Guillaume Dupuytren (1777-1835) published on melanosis too in the same Bulletin.[10,11]

Figure 1:
Charles IV of Spain and His Family, by Francisco Goya (1746-1828), painting 1800-1801, Prado Museum, Madrid. Goya portrays a woman (fourth person from the left) with a concerning pigmented lesion, characterized by an irregular, black plaque with raised edges on her right temple, melanoma seems the most likely case.
Historical recursion on various descriptions of skin cancer
English physician and anatomist Nathaniel Highmore (1614-1685), Danish anatomist Thomas Bartholin (1616-1680) and Genovese Theophile Bonet (1620-1689) reported on various cases of “fatal black tumor”.[12–14] In 1775, Pervicall Pott (1714-1788) made surgical observations relative to the cancer of the scrotum. He was the first scientist to demonstrate that a cancer may be caused by environmental carcinogen. He identified soot as the cause of chimney sweeps scrotal cancer, later called testicular cancer.[15] In 1820, William Norris was the figure to describe the first instance of melanoma and mentioned the relation of melanoma with heredity. He surgically removed a mole of a 59 years old male between umbilicus and the pubes. Unfortunately, the lesion recurred 6 weeks later. The father of this man died of a similar disease about 30 years ago and the youngest son had the same sign in the same spot where the disease in his father initially appeared.[16] French anatomist Gilbert Breschet (1784-1845) made during 1821 a series of clinical observation on melanoma, stating that is a disease appearing to other species too, mentioning the dog, the cat, the rabbit, the rat, the mouse and the horse.[17] In 1834, David Williams described a melanoma as a shoulder’s black spot, developing first horizontal and then vertical.[18,19] Robert Carswell (1793-1857), professor of pathology at the University College of London, described the post-mortem findings of an old man brought to the Hotel Dieu of Paris (Figure 2).[20] In 1838 Isaac Patrish documented the first North American case of melanoma which was a tumor on the foot. In 1838, Scottish expert in pathological anatomy Robert Carswell (1793-1857) introduced the term melanoma in the medical nomenclature in his book “Illustrations of the elementary forms of disease”. He classified the lesions of melanoma in true melanosis and spurious melanosis. He illustrated melanoma metastasis form a cutaneous melanoma of a 70 years old male patient with metastasis in the brain and Ileum after autopsy.[18,19,21,22] In 1844, English surgeon Samuel Cooper (1780-1848) who was coined with the term “melanotic cancer”, highlighted that “the only chance for cure of melanoma depend upon the early removal of the disease, so that to prevent any metastasis”.[19] In 1853, James Paget (1814-1899) recorded 25 cases of “melanoid cancer” and noted once more the development of superficial to vertical spread of melanoma. In 1857, Jonathan Hutchinson (1828-1913), a British surgeon who described melanotic freckle, which is now known as lentigo maligna. In 1885, melanuria was described by Tennent.[23] In 1858, Oliver Pemberton (1825-1897) stated the color changes that a melanoma causes in the skin (Figure 3). He had also recorded that the available on the era treatments were ineffective.[24,25] Surgeon Maurice Henry Collins (1824-1869) in 1864 classified melanosis in fibrinous or simple, cancerous and cystic, noting that not all cases belong to cancer or all fatal (Figure 4).[26] Ten years after Pemberton, in 1868, German pathologist, physiologist and biologist Paul Langerhans (1847-1888) published a description of “branched cells of the skin” that stained with gold chloride.[27] American surgeon John Ashhurst (1839-1900) in 1871 noted that melanotic cancer usually occurs as a separate mass instead of an infiltration, having as favorite localities the skin and the subcutaneous tissue.[28] In 1873, Thomas Bryant (1828-1914) suggested that melanotic cancer always grows from a part which naturally contains pigment.[29] Scottish pathologist William Aitken (1825-1892) said that melanotic cancer consists of encephaloid or soft cancer, with the addition of black or brownish pigment.[30] In 1886, American physician Austin Flint (1812-1886) suggested that most melanotic cancers were in reality melanotic sarcomata.[31] In 1887 German Dieterich P. gave a detailed statistical review of 145 patients reported between the years 1864 to 1881.[32] In the 20th century, advances in microscopy, pathology, immunology, genetics, and molecular biology enabled more accurate and detailed diagnosis of melanoma. In 1903, Eve Frederick Samuel (1853-1916) advocated in far or of wide excision and elective regional node dissection. In 1905, William Sampson Handley (1872-1962) recommended the excision of subcutaneous fat of melanoma for about 2 inches in all directions. In 1911, Rous Sarcoma Virus (RSV), a retrovirus that causes tumors in chickens had been used as a model for studying cancer. The discovery or RSV by American pathologist at the Rockefeller University Francis Peyton Rous (1879-1970) in 1910 opened the field of tumor virology and led to the discovery of oncogenes.[25]
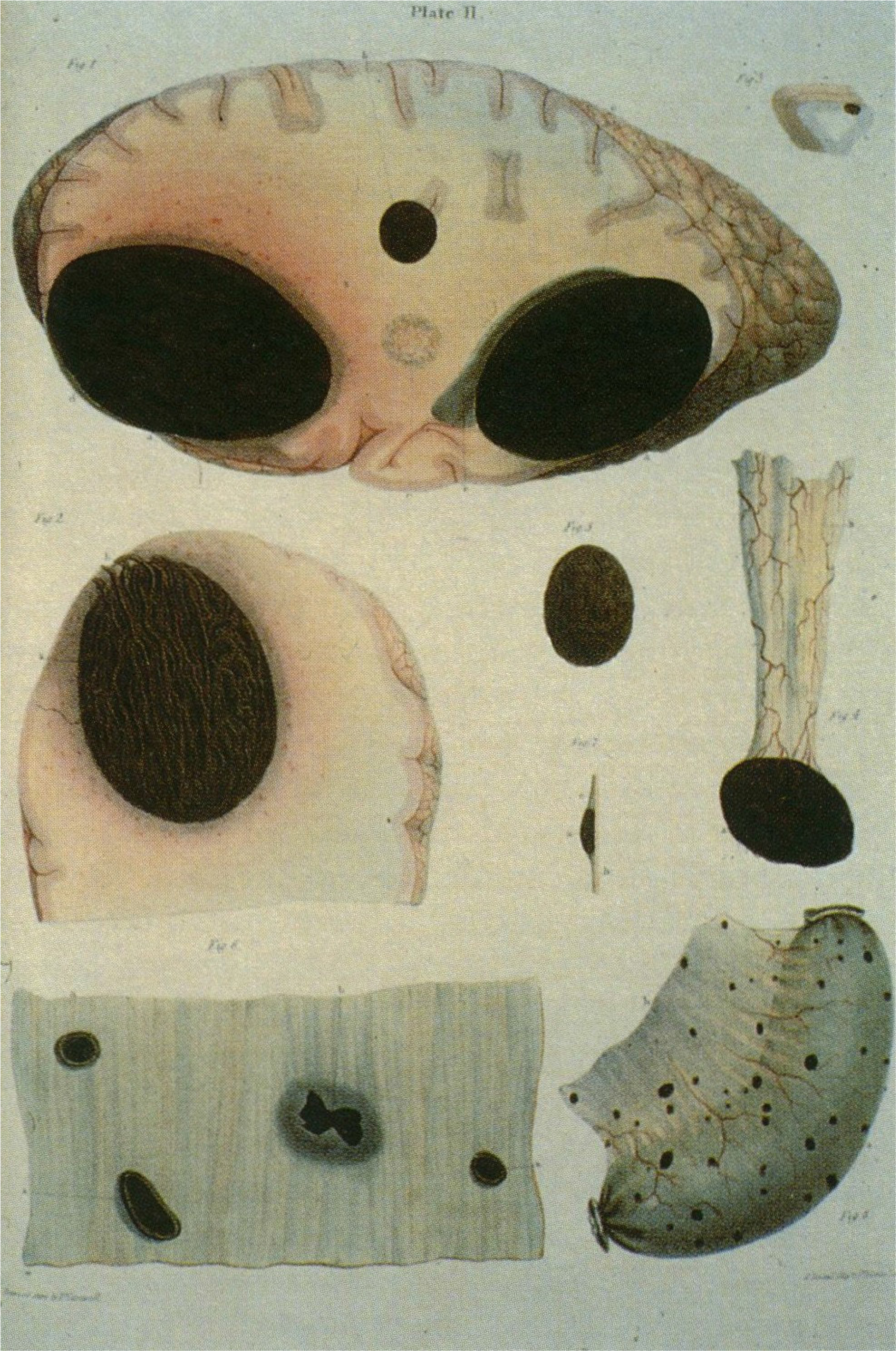
Figure 2:
Breschet’s illustrations of post-mortem finding of a metastatic melanoma case, from within “Considerations sur une alteration organique apelde degenerescence noire milanose, cancer milani”, 1821.
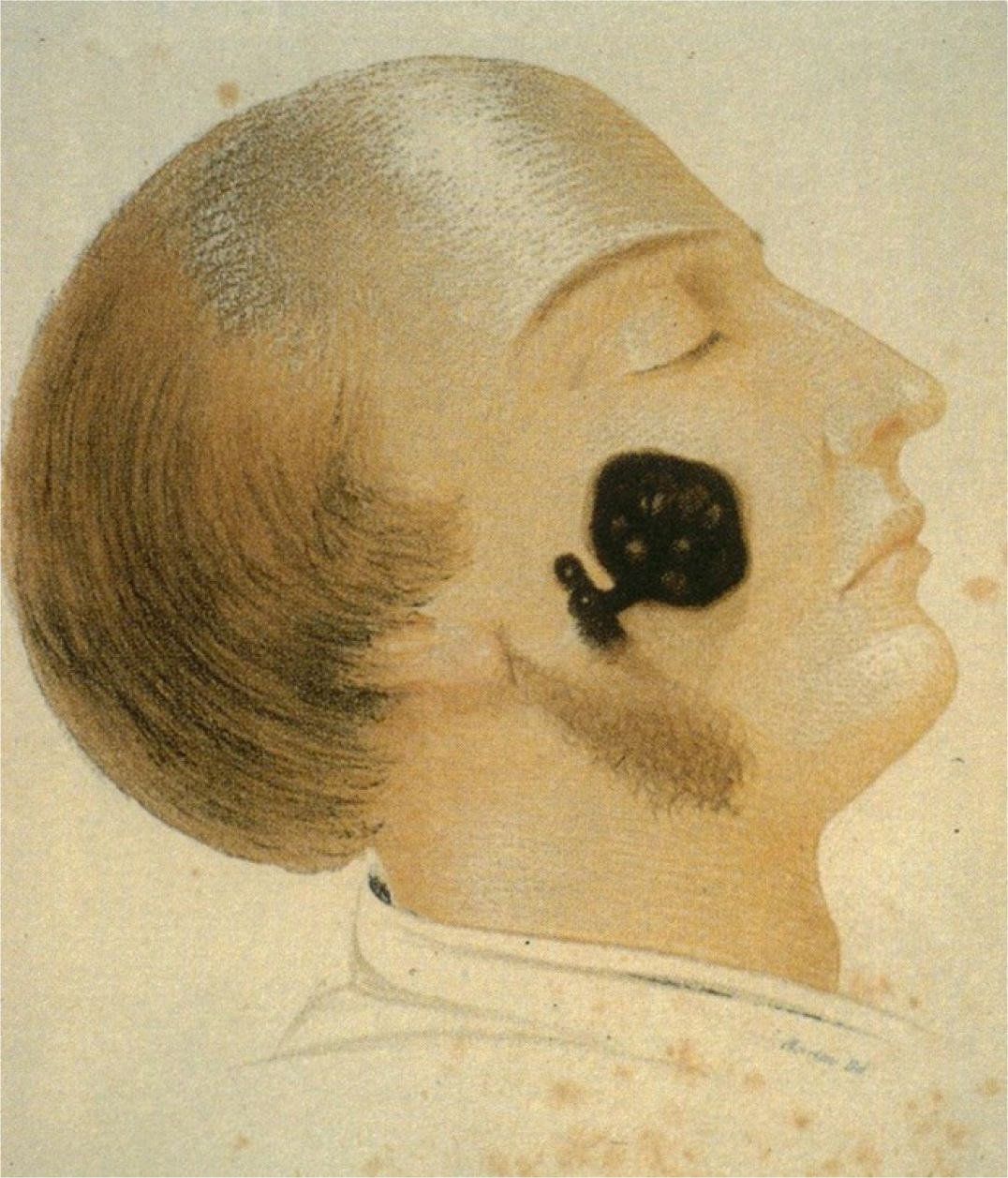
Figure 3:
Melanoma of the face in a 53 years old male from Pemberton’s on Melanosis, 1858.
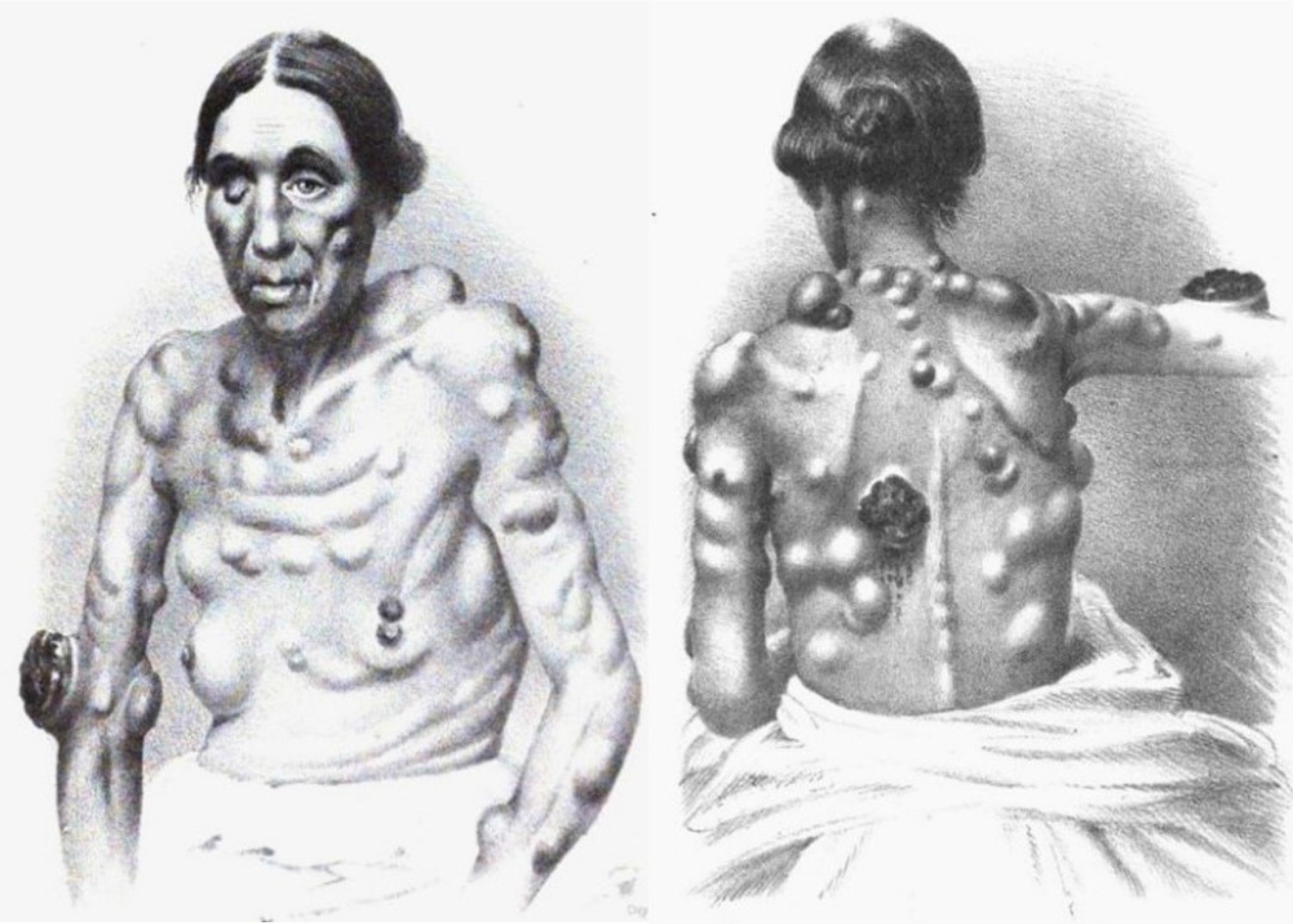
Figure 4:
Female patient with melanotic cancer from Maurice Henry Collins’ on the diagnosis and treatment of cancer and the tumours analogous to it, 1864.
Surgeon and historian Walter George Spencer (1858-1940) from the Westminster Hospital, delivered in November 1923 the Bradshaw Lecture “On melanosis”, reviewing current thinking on the chemical formation of melanin, adding though the in vogue clinical management of the disease.[33] In 1956, Henry Lancaster made a series of observations in Caucasians and reported the relation of sunlight and the incidence of melanoma. Nelson and Lancaster exposed the interaction of 5 skin characteristics, skin color, texture, hair color, eye color and reaction to sun in melanoma expression.[25] Wallace Clark (1924-1997) was an American dermatologist and pathologist who is best known for the description of the “Clark’s level”, or “Clark level system” for classifying the seriousness of a malignant melanoma skin cancer based on its microscopic appearance. The method measures the depth of penetration of a melanoma into the skin according to anatomic layer, categorizing 5 Clark levels of invasion.[20] Alexander Breslow was a pathologist at George Washington University medical center who first reported depth of invasion as a prognostic factor in melanoma in 1970. In recognition of his contribution, the depth of invasion of melanoma is referred to by the eponym Breslow’s depth.[34] Dr. Donald L. Morton was a pioneer in the field of oncology and made significant contributions to the management of cancer patients worldwide. One of his major contributions was the development of the sentinel node biopsy for melanoma, which improved the staging of the disease in 1977. Morton and his colleagues at UCLA medical center developed the first lymphoscintigraphy technique using radio-labeled colloidal gold.[35]
Treating melanoma
Early times
The term melanoma was widely used by Sir Robert Carswell (1793-1857) since around 1838, when the British pathologist studied the microscopic features of various tumors. In 1826, Thomas Fawdington (1795-1843), another British surgeon, wrote that the medical profession was “quite in the dark” as to the causes and treatment of melanoma. The first landmark in melanoma treatment was the recognition of the importance of the surgical excision. In 1892, William Sampson Handley, once more a representative of the British school, proposed that melanoma should be removed with a wide margin of normal skin to prevent local recurrence and metastasis. He had also suggested that lymphatic vessels should be ligated to prevent lymphatic spread. This principle is still followed today, although the optimal width of the margin depends on the thickness and location of the melanoma. Surgery is usually the main treatment for early-stage melanomas, and may also be used to remove lymph nodes or distant metastases in some cases.[7,9,36]
Surgery in melanoma
Surgery is one of the main treatments for melanoma, as it can remove the tumor and prevent it from spreading to other parts of the body. The history of surgery for melanoma dates back to the late 18th century, when the first successful excision of a melanoma tumor was performed. The first man recorded to operate on a patient with melanoma was John Hunter, who performed the first successful excision of such a tumorous entity in 1787, a recurrent mass from an angle of the lower jaw of a 35 years old man, a tumor which was later proven to be melanoma. At the time, he didn’t know exactly what it was and described it as a “cancerous fungous excrescence”. In the 19th century, more and more surgeons recognized melanoma as a distinct disease and advocated for early removal of the tumor with the surrounding tissue as well (Figure 5). In 1851, A. Fergusson performed the first groin radical dissection for lymph nodal metastases, which are common areas of melanoma spread. In the 20th century, surgery for melanoma became more refined and precise, thanks to advances in technology and pathology.[7,9,10,37] In 1892, Herbert Lumley Snow from London, supported the excision and anticipatory gland excision, by suggesting that a regional lymphadenectomy of the regional lymph nodes which function as “traps” of the malignant cells, may prevent the spread of cancer into the blood.[38] One of the most notable innovations was the “Mohs micrographic surgery”, developed by Frederic Edward Mohs (1910-2002) in the 1930s. This technique involved removing thin layers of tissue and examining them under a microscope until no cancer cells are detected. This allows for minimal tissue loss and maximal tumor clearance.[39] During 1960s, the cases of invasive melanomas were considered as high-risk entities in oncological surgery requiring extensive local resection of 5-cm margins of normal skin and subcutaneous tissue in all directions and requiring split-thickness skin graft for wound closure, combined with elective lymph node dissection. Today, surgery for melanoma is still a standard treatment option, especially for early-stage tumors. Depending on the size, location, and depth of the tumor, different types of surgery may be performed, such as simple excision, wide local excision, sentinel lymph node biopsy, lymph node dissection and/or skin grafting.[9,10]
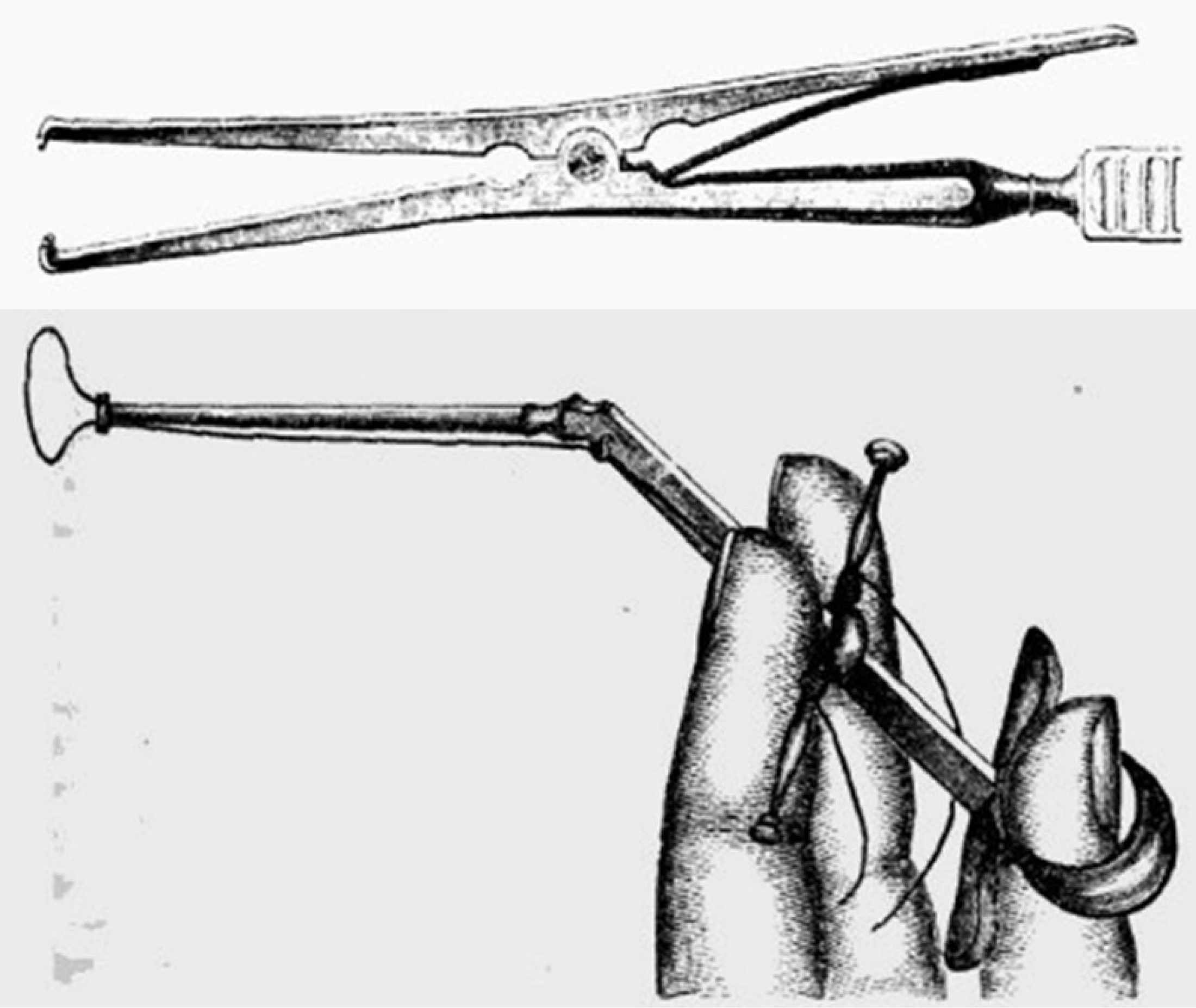
Figure 5:
19th century surgical tools for the removal of melanotic tumors from Robert Druitt’s The Surgeon’s vade mecum, 1856.
Chemotherapy Era
During 1960’s and 1970’s a non-surgical option, called chemotherapy, appeared in the foreground. Creech, Krementz, Ryan and Winblad used perfusion chemotherapy for melanomas of the extremities, utilizing an extracorporea] circuit.[40] Melphanan (1-phenylalanine mustard) had been used by Kremetz and Ryan, with not any effective results for the treatment of metastatic melanoma.[41] The first systemic treatment for melanoma was the chemotherapy option with dacarbazine, a drug which gained FDA approval in May 1975, presenting partial response at median survivals of 5-11 months and an overall of 1 year survival of the 27% of the cases. Dacarbazine is an alkylating agent that damages DNA and induces cell death. However, dacarbazine has low response rates (10-20%) and does not improve survival in most patients with melanoma. Other chemotherapy drugs such as temozolomide, cisplatin, vinblastine, and carmustine have also been used for melanoma, but with similar or even worse results. Chemotherapy was often combined with immunotherapy agents such as interferon-alfa or interleukin-2 (IL-2), which are cytokines that stimulate the immune system to fight cancer cells. However, these combinations did not show significant benefits over chemotherapy alone, and had severe side effects such as fever, chills, fatigue, and various organ toxicity.[42]
Immunotherapy Era
German physician Paul Ehrlich (1854-1915) introduced the theory that the human immune system could repress a potentially “overwhelming frequency” of carcinomas by defending itself.[43] In late 1960’s the concept of the “interference phenomenon” appeared and the role of interferons in antitumor immunity was emphasized by Gresser and Bourali.[44] Steven A. Rozenberg and his team of the National Cancer Institute developed the first effective immunotherapy for metastatic melanoma in the 1980’s. The treatment involved removing white blood cells from patients’ bodies and growing them in a lab with interleukin-2, a protein that stimulates the immune system. The cells were then infused back into the patients’ bodies. The treatment was successful in some patients. In 1974, Golub and Morton described the presence of melanoma antigens that was introduced the clinical studies of vaccines with genetically defined epitopes. Luis F. Parada was one of the researchers who first identified NRAS mutations in melanoma in 1984, along with Robert A. Weinberg and others and published their findings in the journal “Nature”. Nowadays, it is known there are NRAS mutations in 20% of all melanomas. In 1998, high-dose bolus interleukin-2 approved from FDA, especially applied in metastatic melanoma. In 2002, Helen Davies and colleagues were the first to discover high frequency BRAf mutations in human cancers, using a systematic genetic screen. They found that BRAf mutations were present in about 8% of samples, being included in 66% of melanoma cases.[45,46] Genomics paved the way for debouching of 3 melanoma drugs in 2011, approved by the FDA, i) ipilimumab, a monoclonal antibody that activates the immune system to fight cancer cells, ii) vemurafenib, a targeted therapy that blocks a mutated form of a protein called BRAF that drives melanoma growth and iii) peginterferon alfa-2b, a cytokine that stimulates the immune system and inhibits tumor growth. Targeted therapy drugs such as vemurafenib, dabrafenib, trametinib, cobimetinib, and binimetinib inhibit the BRAF or MEK enzymes, blocking the signaling pathway that promotes cancer growth. These drugs have shown remarkable results in improving response rates between 40-70%, progression-free survival between 6-12 months and overall survival up to 18-36 months in patients with advanced melanoma. In 2014, two more drugs were approved by the FDA for advanced or metastatic melanoma, pembrolizumab and nivolumab, immunotherapeutics which prevent cancer cells from evading the immune system. These drugs were also shown to improve survival and progression-free survival in clinical trials.[47]
Prognosis
Prognosis is the likely outcome or course of a disease, such as melanoma. It is usually based on factors such as the stage, type, location, and size of the tumor, as well as the patient’s age, general health, and response to treatment. Prognosis is often expressed as survival rates, which are the percentage of people who survive for a certain period of time after diagnosis. The history of prognosis of melanoma is marked by several events that have improved the survival rates and quality of life of patients with this disease.[4]
In 1978, Clark and colleagues proposed a staging system for melanoma based on the thickness and ulceration of the tumor, as well as the presence or absence of lymph node or distant metastases. This system helped to classify melanoma into different stages and predict the prognosis and treatment options for each stage. In 1985, Alexander Breslow and colleagues introduced a more refined measurement of tumor thickness called “Breslow’s depth”, which is the distance from the top of the granular layer of the epidermis to the deepest point of tumor invasion. Breslow’s depth is one of the most important prognostic factors for melanoma, as thicker tumors tend to have a worse outcome than thinner ones. In 1992, Balch and colleagues revised and updated the staging system for melanoma based on new data and criteria, such as Breslow’s depth, mitotic rate, sentinel lymph node status, and serum lactate dehydrogenase level. This system is still widely used today, to define the stages of melanoma from 0 to 4. These events, alongside the introduction of immunotherapy have contributed to a significant decline in melanoma mortality rates over the past decade by about 5% per year in adults younger than the age of 50 and 3% per year in those of 50 and the elder. However, prognosis still varies widely depending on the stage of melanoma at diagnosis.[4,7,9,48]
The future
Despite continuous advances in melanoma research a cluster of challenges render its therapeutics at least an arduous field, while the tumor itself presents the ability to strongly resist treatments and the capacity to rapidly spread to other organs. Various options aim to confront melanoma, like 5-aminolevulinic acid photodynamic therapy which is an emerging therapeutic strategy for skin cancer due to its noninvasiveness and high spatiotemporal selectivity.[49] It seems though that the key for the treatment approach of melanoma may be in synergy of immunotherapy and Chimeric Antigen Receptor (CAR), while new mRNA vaccines which are the latest trend in oncology therapy, are now exploiting the ability of the human immune system to recognize and destroy cancerous cells.[50]
CONCLUSION
Melanoma remained a poorly understood and fatal condition until up the 20th century, when astute clinical observations and advances in molecular biology revealed the genetic and environmental factors involved in its development and progression. Today, melanoma is one of the most common and aggressive forms of skin cancer, but also one of the most treatable if detected early. Genomics revolutionized studying mutational profiles. Great attainments in melanoma history between 1800’s and up to 2010’s were followed by immunotherapies or cytotoxic chemotherapies. Genetics may offer a brighter future for melanoma and alleviate somehow the agony of oncologists when they treat mostly fatal cases.
References
- Moses OSJ. Melanotic Sarcoma and “Sarcomatous Melanoma”. Ind Med Gaz. 1907;42(6):218-220. [Google Scholar]
- Fawdington T. A Case of Melanosis, with General Observations on the Pathology of the Interesting Disease. Longman, Orme, Brown, Robinson and Bent, London. 1826 [Google Scholar]
- Skarlatos B. Lexicon of the daily spoken language of Greek with interpretation in ancient Greek and French. Andreas Koromblas, Athens. 1874 [Google Scholar]
- . Sunlight, Vitamin D and Skin Cancer. Advances in Experimental Medicine and Biology. 2020;1268 [Google Scholar]
- Brenn T. Histologisches Spektrum des malignen Melanoms [Histological spectrum of malignant melanoma]. Pathologe. 2015;36(1):53-61. [Google Scholar]
- Rebecca VW, Sondak VK, Smalley KS. A brief history of melanoma: from mummies to mutations. Melanoma Res. 2012;22(2):114-122. [Google Scholar]
- . Holland-Frei Cancer Medicine. 2003 [Google Scholar]
- Mihulecea CR, Iancu GM, Leventer M, Rotaru M. The Many Roles of Dermoscopy in Melanoma Detection. Life. 2023;13(2):477 [Google Scholar]
- McLeod GR, Davis NC. Historical overview of melanoma. Clin Dermatol. 1992;10(1):5-7. [Google Scholar]
- Lee C, Collichio F, Ollila D, Moschos S. Historical review of melanoma treatment and outcomes. Clin Dermatol. 2013;31(2):141-147. [Google Scholar]
- Laennec RTH. Sur les melanoses. Bull Faculte Med Paris. 1812;1:24-26. [Google Scholar]
- Gordon JE. Nathaniel Highmore, physician and anatomist, 1614-1685. Practitioner. 1966;196(176):851-858. [Google Scholar]
- Thomas Bartholin (1616-1680): Danish anatomist and his cardinal contributions towards the discovery of the lymphatic system. Eur J Anat. 2017;21(4):261-268. [Google Scholar]
- Theophile Bonet (1620-1689), physician of Geneva. JAMA. 1969;210(5):899 [Google Scholar]
- Brown JR, Thornton JL. Percivall Pott (1714-1788) and chimney sweepers’ cancer of the scrotum. Br J Ind Med. 1957;14(1):68-70. [Google Scholar]
- Silvers DN, Gorham JD. Observations on a melanoma by William Norris, MD, a country practitioner of the early 19th century. Am J Dermatopathol. 1982;4(5):421-424. [Google Scholar]
- Breschet G. Considerations sur une alteration organique apelde degenerescence noire milanose, cancer milani etc. Bechet jeune, Paris. 1821 [Google Scholar]
- Urteaga B, Pack GT. On the antiquity of melanoma. Cancer. 1966;19:607-610. [Google Scholar]
- Cooper S. First lines of the theory and practice of surgery. Longman, Orme and Co, London. 1840 [Google Scholar]
- Carswell R. Melanoma in Illustrations. Am Jf Med Sci. 1838;20:266-268. [Google Scholar]
- Carswell R. Illustrations of the elementary forms of disease. 1838 [Google Scholar]
- Karamanou M, Liappas I, Stamboulis E, Lymperi M, Kyriakis K, Androutsos G, et al. Sir Robert Carswell (1793-1857): coining the term “melanoma”. J BUON. 2012;17(2):400-402. [Google Scholar]
- Scolyer RA, Long GV, Thompson JF. Evolving concepts in melanoma classification and their relevance to multidisciplinary melanoma patient care. Mol Oncol. 2011;5(2):124-136. [Google Scholar]
- Pemberton J. On Melanosis. 1858 [Google Scholar]
- Mcleod GR. Historical Overview of Melanoma. Clinics in Dermatology. 1992;10:5-7. [Google Scholar]
- Collins MH. On the diagnosis and treatment of cancer and the tumours analogous to it. 1864 [Google Scholar]
- Langerhans P. Ueber die Nerven der menschlichen Haut. Arch Pathol Anat Physiol Klin Med. 1868;44:325-337. [Google Scholar]
- Ashhurst J. The Principles and Practice of Surgery. 1871 [Google Scholar]
- Bryant T. The Practice of Surgery. 1873 [Google Scholar]
- Aitken W. The Science and Practice of Medicine. 1883 [Google Scholar]
- Flint A. A Treatise on the principles and practice of medicine. 1886 [Google Scholar]
- Dieterich P. Ein Beittag zur Statistik und Klinischlen Bedeutung Melanotischer Geschwulste. Langenbeck’s Arch Klin Chir. 1887;35:289-320. [Google Scholar]
- Spencer WG. On melanosis. Br Med J. 1923;2:907-913. [Google Scholar]
- . Cutaneous Melanoma. 2020 [Google Scholar]
- Balch CM, Roh MS, Suzanne Klimberg V. In Memoriam: Donald L. Morton, MD (1934-2014): An Icon in Surgical Oncology. Ann Surg Oncol. 2014;21:1413-1416. [Google Scholar]
- Abdalla S, Ellis H. William Sampson Handley (1872-1962): champion of the permeation theory of dissemination of breast cancer. J Med Biogr. 2013;21(2):108-111. [Google Scholar]
- Fergusson A. Recurrence of a melanotic tumour; removal. Lancet. 1851;57(1449):622 [Google Scholar]
- Snow H. Melanotic cancerous disease. Lancet. 1892;2:872 [Google Scholar]
- Ross NA, Saedi N, Yeo CJ, Cowan S. Frederic E. Mohs, M.D. (1910-2002): physician and innovator. Am Surg. 2015;81(5):433-437. [Google Scholar]
- Creech O, Krementz ET, Ryan RF, Winblad JN. Chemotherapy of cancer: Regional perfusion utilizing an extratcorporeal circuit. Ann burg. 1958;148:616 [Google Scholar]
- Litwin MS, Ryan RF, Ichinose H, Reed RR, Kremetz ET. Proceedings: Use of 5 fluorouracil in the topical therapy of skin cancer: a review of 157 patients. Proc Natl Cancer Conf. 1972;7:549-561. [Google Scholar]
- Velho TR. Metastatic melanoma – a review of current and future drugs. Drugs Context. 2012;2012 [Google Scholar]
- Ehrlich P. Ueber den jetzigen stand der Karzinomforschung. Ned Tijdschr Geneeskd. 1909;5:273-290. [Google Scholar]
- Gresser I, Bourali C. Exogenous interferons and inducers of interferon in the treatment of Balb-c mice inoculated with RC19 tumour cells. Nature. 1969;223:844-845. [Google Scholar]
- Zhang Y, Zhang Z. The history and advances in cancer immunotherapy: understanding the characteristics of tumor-infiltrating immune cells and their therapeutic implications. Cell Mol Immunol. 2020;17(8):807-821. [Google Scholar]
- Dobosz P, Dzieciątkowski T. The Intriguing History of Cancer Immunotherapy. Front Immunol. 2019;10:2965 [Google Scholar]
- Eggermont AM, Robert C. New drugs in melanoma: it’s a whole new world. Eur J Cancer. 2011;47(14):2150-2157. [Google Scholar]
- Keller D, Hundeiker M. Neue Gesichtspunkte zum “prognostischen Index” bei malignen Melanomen [New viewpoints on the prognostic index in malignant melanomas]. Z Hautkr. 1985;60(3):279-282. [Google Scholar]
- Wang Y, Fu S, Zeng Y, Jiao S, Chai G, Xu Y, et al. Tea polyphenols nanoparticles integrated with microneedles multifunctionally boost 5-aminolevulinic acid photodynamic therapy for skin cancer. J Colloid Interface Sci. 2024;677((Pt A)):446-458. [Google Scholar]
- Sorino C, Iezzi S, Ciuffreda L, Falcone I. Immunotherapy in melanoma: advances, pitfalls, and future perspectives. Front Mol Biosci. 2024;11 [Google Scholar]



