ABSTRACT
SUMMARY
Purpose
Cervical cancer is a prevalent gynecological malignancy that leads to mortality in women residing in underdeveloped nations. Precise diagnostics are essential for achieving a more favorable outcome, particularly in the later stages of the disease. This study aims to evaluate and compare the efficacy of ultrasonography and cystoscopy in identifying bladder involvement in patients with advanced cervical cancer.
Methods
A prospective study was conducted between January 2019 and June 2023 on 19 patients with advanced cervical cancer. The patients were examined with both ultrasonography and cystoscopy to diagnose bladder involvement. Subsequently, the patients were assessed for the presence or absence of bladder involvement using an ultrasound scan and a cystoscopy.
Results
10 out of 19 patients showed bladder involvement on cystoscopy, while the others showed no bladder involvement on cystoscopy. Out of the 13 patients with clinical non-hematuria, nine show cystoscopy with no bladder involvement. Ultrasonography showed bladder involvement in 17 cases. Therefore, in this study, ultrasound was 89% sensitive in detecting bladder involvement in cervical cancer.
Conclusion
In advanced cervical cancer, ultrasonography may identify bladder invasion more precisely than cystoscopy.
INTRODUCTION
Cervical cancer is a common gynecological cancer that results in death among women living in developing countries.[1] In 2022, around 662,301 new cases of cervical cancer were detected, resulting in 348,874 deaths globally as a result of this malignant condition.[2] Indonesia ranks fourth among Southeast Asian countries in terms of cervical cancer incidence, following Cambodia, Myanmar, and Thailand.[3] Human Papillomavirus (HPV) causes 90% of cervical cancer with two types of viruses such as types 16 and 18, and is responsible for 70% of all cervical cancers.[4,5]
The treatment is determined based on The International Federation of Gynecology and Obstetrics (FIGO) staging.[1,6] Approximately 6–8% of cervical cancer patients show invasion of the urinary bladder.[1] FIGO has advised the use of Intravenous Pyelogram (IVP) and cystoscopy as the preferred methods for staging cervical cancer.[7] However, previous studies have shown FIGO staging to be relatively inaccurate in some cases.[8] Cystoscopy may not detect bladder invasion if the tumour only affects the outer bladder wall and does not invade the mucosa.[1] Several alternatives to traditional methods of bladder invasion detection have been developed, such as Magnetic Resonance Imaging (MRI) and Computed Tomography (CT) scans, their widespread availability and patient affordability continue to be major obstacles.[1,9] Another modality, such as laparoscopy, could be used but this is also an invasive procedure that allows macroscopic inspection of tumor involvement and biopsy samples from suspicious sites at the cervicovesical junction.[9]
Ultrasonography (US) appears to be an appropriate alternative for diagnosing bladder involvement.[1,6] Because of its limited contrast resolution, it has been reported to be ineffective in pretreatment evaluations of invasive cervical cancer.[10] However, new high-resolution US provides a rather convincing representation of the tissue planes between the cervix and the lower urinary tract.[9,10] Ultrasonography is a promising tool for diagnosing bladder invasion due to its ease of use, non-invasive nature, and low cost. Bladder invasion can be readily identified with US by noting any disruption, irregularity, or tenting of the outer wall of the bladder (Figure 1).[10] Therefore, this study aimed to evaluate and compare the efficacy of ultrasonography and cystoscopy in identifying bladder involvement in patients with advanced cervical cancer.
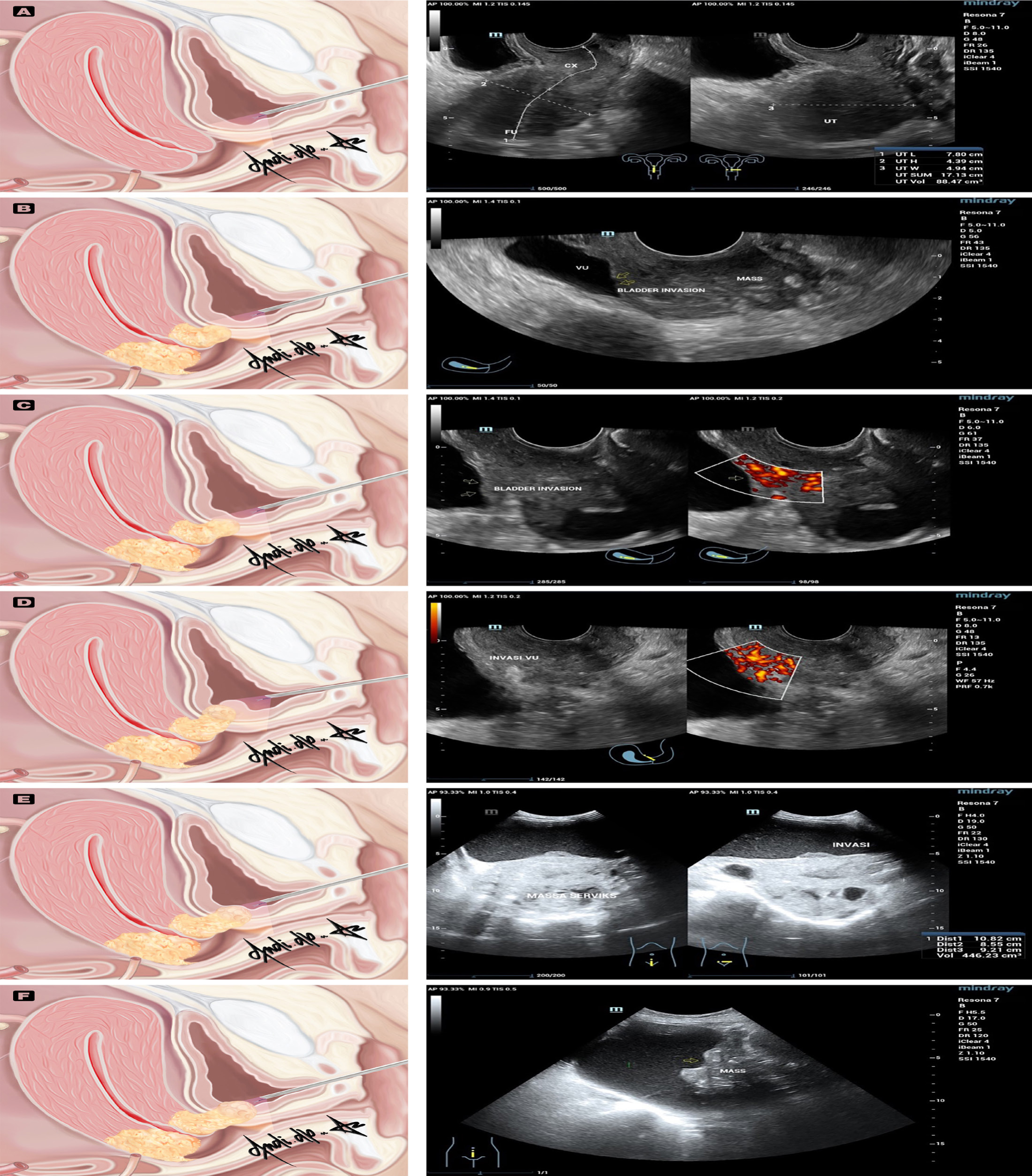
Figure 1:
Anatomical variations seen in cystoscopy of bladder metastases in cervical cancer (left) compare to ultrasonography (right). Figure A normal cystoscopy (left) and normal TVUS (right); Figure B, C, D cystoscopy is unable to reach the tumor site for biopsy as the tumor location is unknown (left), while TVUS could reach the tumor site (right). Figure E and 1F images from a bladder cystoscopy allowed for the biopsy of the tumor (left), and the detection of bladder invasion on TAUS (right). UT, uterus; FU, fundus uteri; CX, cervix; VU, vesica urinaria. Anatomical variations seen in cystoscopy of bladder metastases in cervical cancer (left) compare to ultrasonography (right). Figure A normal cystoscopy (left) and normal TVUS (right); Figure B, C, D cystoscopy is unable to reach the tumor site for biopsy as the tumor location is unknown (left), while TVUS could reach the tumor site (right). Figure E and 1F images from a bladder cystoscopy allowed for the biopsy of the tumor (left), and the detection of bladder invasion on TAUS (right). UT, uterus; FU, fundus uteri; CX, cervix; VU, vesica urinaria.
METHODS
A single prospective study was conducted between January 2019 and June 2023 at Cipto Mangunkusumo Hospital. The study enrolled all female patients aged 18 years and older, all of whom had histopathologically confirmed stage IV cervical cancer based on the revised 2018 International Federation of Gynecology and Obstetrics (FIGO) staging. The inclusion criteria required that participants be clinically suspected of having metastasis to the urinary bladder and undergo both Transabdominal Ultrasound (TAUS) or Transvaginal Ultrasound (TVUS) and cystoscopy as part of their diagnostic evaluation. Exclusion criteria included patients with a history of other primary malignancies involving the urinary bladder, prior bladder surgery that could interfere with diagnostic accuracy, incomplete medical records, and patients who declined to participate or withdraw consent during the study. All patients signed informed consent prior to examination. A single oncologist performed the procedure to eliminate any interobserver variations.
Ultrasonography was performed with patients in the supine position using Mindray Resona 7 (Shenzhen, China). The US system uses 2D TVUS equipped with a 5-7 MHz endovaginal transducer and TAUS equipped with a 4-5.5 MHz abdominal transducer. Intratumoral flow was assessed using power Doppler. The images were optimized using time gain compensation setting. During the TVUS procedure, the transducer was positioned in the anterior fornix in order to focus on the cervicovesical junction, while during the TAUS procedure, the transducer was positioned in the lower abdomen. The images were captured in longitudinal and transversal planes to detect any structural alterations that might indicate bladder invasions, such as bladder wall tenting and disruption of the bladder-cervix interface (Figure 2). The patients also had cystoscopic exams concurrently to compare the findings. The patients were evaluated at three different stages of tumor invasion.[11] Grade I: limited involvement of the external hyperechogenic layer (possibly associated with the vesicular fascia). Grade 2: Infiltration, which extends into the deeper hypoechogenic muscle layer. Grade 3: The growth reaches the hyperechogenic mucosa and manifests as intraluminal tumor nodules.
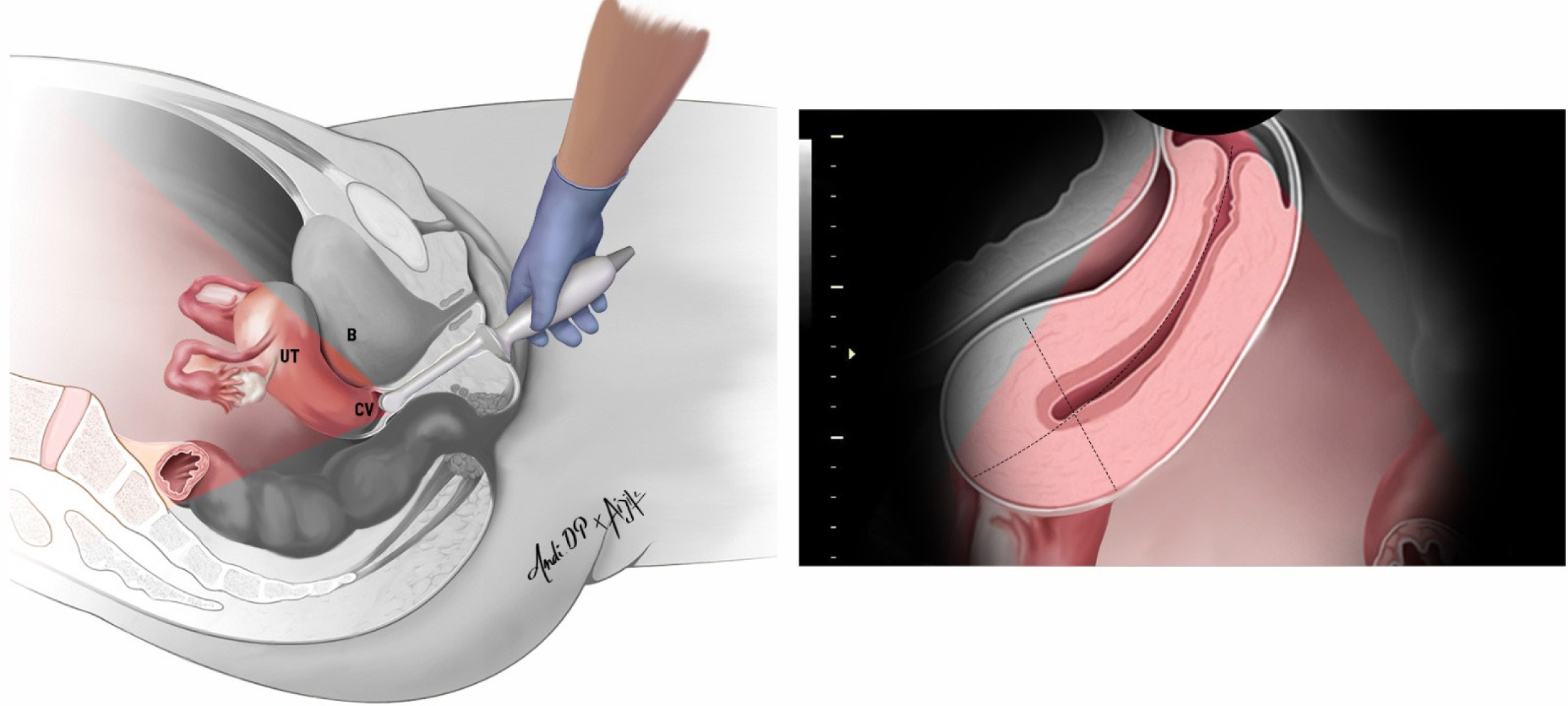
Figure 2:
A transvaginal transducer is inserted into the anterior fornix of the vagina and used to study the bladder wall in the sagittal plane. UT: uterus; CV: cervix, B: bladder.
RESULTS
The study examined a group of women whose ages ranged from 37 to 64 years, with 10 out of the 19 participants being postmenopausal. Histological examination revealed that 18 of the patients were diagnosed with squamous cell carcinoma, a prevalent form of cervical cancer, while one patient had adenocarcinoma, which is less common (as indicated in Table 1). Diagnostic evaluations using both ultrasound and cystoscopy were performed on case number 13, and neither method detected bladder involvement.
| Case (Age) | Post-menopause | Staging | Clinical Hematuria | Ultrasound (Bladder Involvement) | Cystoscopy Primary Site (Bladder Involvement) | Histopathology |
|---|---|---|---|---|---|---|
| Case 1 (37) | – | IVA | + | + | + | SCC |
| Case 2 (42) | – | IVA | + | + | + | SCC |
| Case 3 (47) | – | IVA | + | + | + | SCC |
| Case 4 (45) | – | IVB | + | + | + | SCC |
| Case 5 (61) | + | IVA | – | + | – | SCC |
| Case 6 (64) | + | IVA | + | + | + | SCC |
| Case 7 (41) | – | IVA | – | + | + | SCC |
| Case 8 (49) | + | IVA | – | + | – | SCC |
| Case 9 (64) | + | IVA | – | + | – | SCC |
| Case 10 (63) | + | IVA | – | – | + | SCC |
| Case 11 (57) | + | IVA | – | + | + | SCC |
| Case 12 (53) | – | IVA | – | + | – | SCC |
| Case 13 (53) | + | IVA | – | – | – | SCC |
| Case 14 (44) | – | IVAr | + | + | + | SCC |
| Case 15 (50) | + | IVAr | – | + | – | SCC |
| Case 16 (63) | + | IVAr | – | + | – | AC |
| Case 17 (45) | – | IVAr | – | + | – | SCC |
| Case 18 (48) | – | IVA | – | + | + | SCC |
| Case 19 (62) | + | IVA | – | + | – | SCC |
Regarding disease staging, the study used the FIGO staging system, specifically focusing on Stage IV cervical cancer, which indicates advanced disease. According to FIGO, Stage IV cervical cancer is characterized by the spread of the carcinoma beyond the true pelvis or involvement of the mucosa of the bladder or rectum. Within this classification, Stage IVA signifies that the cancer has extended to adjacent pelvic organs. When imaging confirms this spread, it is classified as Stage IVAr. Stage IVB represents the most advanced stage, where the cancer has metastasized to distant organs.
Among the subjects, 18 patients were diagnosed with Stage IVA cervical cancer, constituting 95% of the cases studied. This includes cases confirmed by imaging, classified as Stage IVAr. Only one patient was found to have Stage IVB cervical cancer, representing 5% of the cases. Hematuria, or the presence of blood in the urine, was detected in 6 cases (32%), while it was absent in the remaining 13 cases (68%). Cystoscopy identified bladder involvement in 53% of the cases, whereas ultrasound showed bladder involvement in 17 cases, representing a significant 89% of the cases (as shown in Table 2).
| Variable | n* | %* |
|---|---|---|
| Staging | ||
| IVA | 14 | 74% |
| IVB | 1 | 5% |
| IVAr | 4 | 21% |
| Histopatology | ||
| SCC | 18 | 95% |
| AC | 1 | 5% |
| Hematuria | ||
| Yes | 6 | 32% |
| No | 13 | 68% |
| Bladder Involvement (US) | ||
| Yes | 17 | 89% |
| No | 2 | 11% |
| Bladder Involvement (Cystoscopy) | ||
| Yes | 10 | 53% |
| No | 9 | 47% |
DISCUSSION
This study includes patients with grade IV cervical cancer according to the 2018 FIGO staging, where the condition involves the infiltration of the mucosal linings of the rectum and bladder, as well as the presence of enlarged lymph nodes in the inguinal and supraclavicular regions. Metastatic cancer has also progressed to other organs within the body, including the lungs and bones.[12] This study focuses on the involvement of the bladder.
Cervical cancer patients with suspected bladder infiltration may undergo a cystoscopy for confirmation.[13] Cystoscopy provides direct visualization of the lower urinary tract anatomy, as well as any macroscopic pathological changes that may underlie the presenting clinical signs. Clinical staging of cervical cancer does not require cystoscopy, particularly in the absence of well-defined clinical signs that suggest bladder involvement.[14] Although cystoscopy offers advantages, it is an invasive procedure that can be time-consuming and costly.[15,16]
Ultrasonography may be used to detect bladder wall involvement with the characteristic of the presence of a tumor protuberance at the cervix-uterine corpus junction. This tumour has invaded the supratrigonal area of the bladder, elevating its wall and giving it a tent-like appearance on transversal ultrasound.[1,9] Furthermore, an irregular surface and echotexture were detected on the inner bladder wall, which was also discovered to be heteroechoic.1 Areas with higher echogenicity or lower echogenicity and an irregular shape suggest alterations that are consistent with cervical cancer.[17] Cervical carcinoma may exhibit an exophytic or an endophytic growth pattern.[18]
The potential of US to provide tumor grading was assessed compared to cystoscopy. The rationale behind this study lies in the possibility that the absence of hematuria might indicate a lack of tumor invasion beyond the mucosal membrane, potentially leading to negative cystoscopy findings. In a previous study conducted in Thailand, painless hematuria may serve as a potential screening tool for identifying patients with cervical cancer who exhibit mucosal infiltration of the urinary bladder.[19] The current study found a difference between the percentage of people in the study population who had hematuria (32%) and those who had bladder invasion in cystoscopic findings (47%). However, tumors in the submucosa may also present with hematuria.[20]
In this study, the sensitivity of US in detecting cervical cancer invasion in the bladder was 89%, which is comparable to previous studies that found 76.2% sensitivity in bladder cancer detection and 95% sensitivity in bladder nodule protrusion detection using ultrasonography.[21,22] Ultrasonography in the sagittal and transversal planes could offer improved visualization of tumor infiltration.[11] The efficacy of ultrasonography in detecting bladder tumors varies depending on tumor size and location. For tumors larger than 0.5 cm located on the lateral or posterior bladder wall, US achieves a high detection rate of 95%. US Doppler can be utilized to assess intratumoral flow, enabling differentiation between tumors and blood clots.[23] In patients with hematuria, US showed relatively high specificity but lower sensitivity in detecting bladder tumors, according to studies. [24,25] Nevertheless, US offers several advantages, including the procedure being cost-effective and tolerable by patients.[24,26]
A separate investigation conducted by Iwamoto K. et al., revealed that CT, MRI, or cytoscopic examination had comparatively lower levels of accuracy (76%, 86%, and 80% respectively) than TVUS (95%) in detecting bladder invasion.[6] Study reported by Arribas S. et al., showed a good agreement between MRI examination and 3D sonography in detecting bladder involvement for local staging of cervical cancer (κ = 0.84; 95% CI, 0.55–1.0; 97.5% agreement) and also found a moderate agreement between MRI examination and 2D sonography (κ = 0.48; 95% CI, 0.10–0.99; 95.0% agreement).[27] Huang WC et al., also found that Transvaginal Sonography (TVUS) has a 100% sensitivity for detecting bladder infiltration.[9] The limitations of this study include the insufficient number of samples, which need to be confirmed by trials with a larger sample size. The ability of US to identify subtle tumor involvement that is not visible on cystoscopy also requires further investigation.
CONCLUSION
This study showed that ultrasonography was 89% sensitive for detecting bladder involvement in cervical cancer. If the tumour merely infiltrates the outer bladder wall without affecting the mucosa, it is possible for bladder invasion to go unreported during a cystoscopic examination. Ultrasonography allows for distinct differentiation and visualization of the inner and outer walls of the bladder.
Cite this article:
Putra AD, Andrijono, Winarto H, Prijanti AR, Lisnawati, Pakasi TA, et al. Ultrasonography versus Cystoscopy for Diagnosing Cervical Cancer Metastasis to Urinary Bladder, Which one is more accurate?. Journal of BUON. 2024;27:14-20.
ACKNOWLEDGEMENT
The author expresses gratitude to the directors of Dr. Cipto Mangunkusumo Hospital for their assistance in the preparation of this article.
ABBREVIATIONS
| CT: | Computed Tomography |
|---|---|
| FIGO: | International Federation of Gynecology and Obstetrics |
| HPV: | Human Papillomavirus |
| IVP: | Intravenous Pyelogram |
| MRI: | Magnetic Resonance Imaging |
| TAUS: | Transabdominal Ultrasound |
| TVUS: | Transvaginal Ultrasound |
| US: | Ultrasound |
References
- Zutshi V, Makkar B, Garg A, Batra S. Transvaginal sonography versus cystoscopy for detecting urinary bladder invasion in early stage cervical cancer. J Clin Diagn Res. 2016;11(2) [Google Scholar]
- Ferlay J, Ervik M, Lam F, Laversanne M, Colombet M, Mery L, et al. International Agency for Research on Cancer. 2024 [cited 2024 Jan 20]. Available fromhttps://gco.iarc.who.int/media/globocan/factsheets/populations/900-world-fact-sheet.pdf
Global Cancer Observatory: Cancer Today. - Nurcahyanti AD. Cervical Cancer: The Case in Indonesia and Natural Product-Based Therapy. J Cancer Biol Res. 2016;4(1):1078 [Google Scholar]
- Pandey U. What is Cervical Cancer?. J Gynecol Women’s Health. 2017;2(5) [Google Scholar]
- Kumar N. Cervical Cancer; a Nightmare for Womanhood: Review of Recent Advances. Womens Health Gynecol. 2016;2(2):17 Available fromwww.scientonline.org
[Google Scholar] - Iwamoto K, Kigawa J, Minagawa Y, Miura H, Terakawa N. Transvaginal ultrasonographic diagnosis of bladder-wall invasion in patients with cervical cancer. Obstet Gynecol. 1994;83(2):217-9. [Google Scholar]
- Fischerova D, Frühauf F, Burgetova A, Haldorsen IS, Gatti E, Cibula D, et al. The Role of Imaging in Cervical Cancer Staging: ESGO/ESTRO/ESP Guidelines (Update 2023). Cancers (Basel). 2024;16(4):775 Available fromhttps://doi.org/10.3390/cancersAttribution
[Google Scholar] - Berek JS, Matsuo K, Grubbs BH, Gaffney DK, Lee SI, Kilcoyne A, et al. Multidisciplinary perspectives on newly revised 2018 FIGO staging of cancer of the cervix uteri. J Gynecol Oncol. 2019;30(2) [Google Scholar]
- Huang WC, Yang JM, Yang YC, Yang SH. Ultrasonographic characteristics and cystoscopic correlates of bladder wall invasion by endophytic cervical cancer. Ultrasound Obstet Gynecol. 2006;27(6):680-6. [Google Scholar]
- Gaurilcikas A, Vaitkiene D, Cizauskas A, Inciura A, Svedas E, MacIuleviciene R, et al. Early-stage cervical cancer: Agreement between ultrasound and histopathological findings with regard to tumor size and extent of local disease. Ultrasound Obstet Gynecol. 2011;38(6):707-15. [Google Scholar]
- Fischerova D. Ultrasound scanning of the pelvis and abdomen for staging of gynecological tumors: A review. Ultrasound Obstet Gynecol. 2011;38(3):246-66. [Google Scholar]
- Salib MY, Russell JHB, Stewart VR, Sudderuddin SA, Barwick TD, Rockall AG, et al. 2018 figo staging classification for cervical cancer: Added benefits of imaging. Radiographics. 2020;40(6):1807-22. [Google Scholar]
- Chopra SJ, Mathew A, Maheshwari A, Bhatla N, Singh S, Rai B, et al. National Cancer Grid of India Consensus Guidelines on the Management of Cervical Cancer. J Glob Oncol. 2018(4):1-15. [Google Scholar]
- Kakinoki Y, Udo K, Tobu S, Noguchi M. The Role of Cystoscopy in The Staging of Cervical Cancer. Jpn J Urol. 2018;109(4):208-15. [Google Scholar]
- Stamatiou K, Papadoliopoulos I, Dahanis S, Zafiropoulos G, Polizois K. The accuracy of ultrasonography in the diagnosis of superficial bladder tumors in patients presenting with hematuria. Ann Saudi Med. 2009;29(2):134-7. [Google Scholar]
- Kalokairinou K, Ploumidis A, Kalogeropoulos T, Vlachos L, Stringaris K, Tavernaraki A, et al. The role of virtual cystoscopy, after multidetector computed tomography imaging reconstruction without the use of contrast medium, in the diagnosis and evaluations of bladder tumors: Preliminary study. Adv Urol. 2014;2014:923958 [Google Scholar]
- Sandra L. Hagen-Ansert. Textbook of Diagnostic Sonography. 2018:1 [Google Scholar]
- Alcazar Juan Luis. Ultrasound Assessment in Gynecologic Oncology. 2018 [Google Scholar]
- Chuttiangtum A, Udomthavornsuk B, Chumworathayi B. Hematuria screening test for urinary bladder mucosal infiltration in cervical cancer. Asian Pac J Cancer Prev. 2012;13(10):4931-3. [Google Scholar]
- Niimi F, Danno T, Iwata S, Honda S, Itagaki S, Azuma T, et al. Submucosal urothelial bladder cancer: A case report. Mol Clin Oncol. 2021;14(4):77 [Google Scholar]
- Barak V, Itzkovich D, Einarsson R, Gofrit O, Pode D. Non-invasive detection of bladder cancer by UBC rapid test, ultrasonography and cytology. Anticancer Res. 2020;40(7):3967-72. [Google Scholar]
- Ros C, de Guirior C, Rius M, Escura S, Martínez-Zamora MÁ, Gracia M, et al. Accuracy of Transvaginal Ultrasound Compared to Cystoscopy in the Diagnosis of Bladder Endometriosis Nodules. J Ultrasound Med. 2021;40(8):1571-8. [Google Scholar]
- Abouelkheir RT, Abdelhamid A, Abou El-Ghar M, El-Diasty T, Cova A. Imaging of Bladder Cancer: Standard Applications and Future Trends. Medicina (Kaunas). 2021;57(3):220 Available fromhttps://doi.org/10.3390/medicina
[Google Scholar] - Mostafaloo M, Ghaemian N, Hajian-Tilaki K, Moudi E. Comparison of bladder ultrasonographic and rigid cystoscopic findings in patients with hematuria. Caspian J Intern Med. 2019;10(4):417-23. [Google Scholar]
- Gharibvand M, Kazemi M, Motamedfar A, Sametzadeh M, Sahraeizadeh A. The role of ultrasound in diagnosis and evaluation of bladder tumors. J Family Med Prim Care. 2017;6(4):840 [Google Scholar]
- Awad M, Harraz AM, Farg H, Gabr HS, Sharaf DE, Abou-El-Ghar M, et al. Microscopic hematuria and pelvic ultrasonography could rule out flexible cystoscopy during surveillance for T1-low grade non-muscle invasive bladder cancer. Arab J Urol. 2023;21(3):150-5. [Google Scholar]
- Arribas S, Alcázar JL, Arraiza M, Benito A, Minguez JA, Jurado M, et al. Three-dimensional transvaginal sonography and magnetic resonance imaging for local staging of cervical cancer: An agreement study. Journal of Ultrasound in Medicine. 2016;35(5):867-73. [Google Scholar]



