ABSTRACT
Objectives:
Combination therapy has emerged as a crucial approach to enhance immunotherapy efficacy in advanced Non-Small Cell Lung Cancer (NSCLC). However, its value in reversing primary immune resistance remains unknown. Therefore, we investigated the efficacy and safety of anlotinib combined with single-agent chemotherapy in patients with advanced primary immune-resistant lung cancer.
Methods:
A total of 35 Advanced Primary Immune-Resistant NSCLC patients were enrolled in this retrospective study over 13 months follow up. The primary endpoints included Progression-Free Survival (PFS), Overall Survival (OS), Objective Response Rate (ORR), Disease Control Rate (DCR), and safety profile are assessed through standardized criteria.
Results:
Our findings demonstrate that the combination of anlotinib and single chemotherapy bring about promising median PFS (4.0 m) and OS (8.0 m) for this refractory population, with an DCR of 65%. Subgroup analysis indicated that the efficacy correlate with the occurrence of some specific Adverse Event such as hypertension, foot-hand syndrome and proteinuria. Mechanistic studies indicate that such regimen may likely exerts its anti-tumor effects by depleting immunue suppression to active immunity function. Beside promising efficacy, such regimen exhibited a manageable safety profile.
Conclusion:
These results support the combination of anlotinib and single chemotherapy with promising efficacy and tolerated toxicity for advanced primary immune resistance NSCLC, offering meaningful clinical benefits with tolerable side effects. Further research is warranted to optimize treatment strategies and explore its potential in combination therapies.
INTRODUCTION
Advanced lung cancer remains a significant global health challenge, with poor prognosis and limited treatment options,[1] especially in cases where tumors exhibit primary resistance to Immune Checkpoint Inhibitors (ICIs).[2] Immune resistance can arise due to various mechanisms, including tumor-intrinsic factors and alterations in the tumor microenvironment, leading to decreased effectiveness of immunotherapy. In recent years, efforts have been directed towards exploring alternative treatment strategies to improve outcomes in this challenging patient population.[3]
Anlotinib hydrochloride (AL3818), a novel multitarget Tyrosine Kinase Inhibitor (TKI), has emerged as a promising therapeutic option in various solid tumors, including lung cancer. It exerts its antitumor effects by targeting Vascular Endothelial Growth Factor Receptor (VEGFR), Platelet-Derived Growth Factor Receptor (PDGFR), Fibroblast Growth Factor Receptor (FGFR), and c-Kit, among others, thereby inhibiting angiogenesis and tumor cell proliferation. In clinical trials, anlotinib has demonstrated efficacy and manageable toxicity profiles in patients with advanced Non-Small Cell Lung Cancer (NSCLC), both as a monotherapy and in combination with chemotherapy.[4,5]
The rationale for combining anlotinib with single-agent chemotherapy in primary immune-resistant lung cancer lies in its potential to synergistically enhance therapeutic efficacy through complementary mechanisms of action.[6] Chemotherapy agents such as paclitaxel, docetaxel, gemcitabine, and pemetrexed are commonly used in the treatment of advanced lung cancer,[7,8] exerting cytotoxic effects on rapidly dividing cancer cells. When combined with anlotinib, which targets angiogenesis and tumor growth pathways, these agents may offer a dual approach to inhibit tumor progression and potentially overcome immune resistance mechanisms.[9]
Despite these potential benefits, the optimal sequencing, dosing, and safety profiles of anlotinib combined with single-agent chemotherapy in primary immune-resistant lung cancer require further elucidation.[9] Previous studies have provided preliminary evidence of efficacy and safety, but larger-scale clinical trials are needed to validate these findings and establish treatment guideline. Moreover, biomarker-driven approaches to identify patient subgroups most likely to benefit from this combination therapy represent an important area of ongoing research.[10] This study aims to contribute to the evolving landscape of treatment options for patients facing immune resistance. By exploring the synergistic potential of anlotinib and chemotherapy, this research seeks to improve outcomes and quality of life for individuals with advanced lung cancer who have limited therapeutic alternatives due to primary immune resistance.
MATERIALS AND METHODS
Study Design
This study is designed as a retrospective, Multicenter, study with the aim to evaluate the efficacy and safety of combining anlotinib with single-agent chemotherapy in patients diagnosed with advanced primary immune-resistant lung cancer. The trial will be conducted in accordance with the principles outlined in the Declaration of Helsinki and Good Clinical Practice guidelines. Eligible patients will be adults aged 18 years or older with histologically confirmed Non-Small Cell Lung Cancer (NSCLC) that is locally advanced or metastatic and has been determined to be primary immune-resistant. Primary immune resistance will be defined as disease progression during or within 3 months of completing treatment with an Immune Checkpoint Inhibitor (ICI), or stable disease as best response without clinical benefit for at least 6 months of ICI therapy. Key inclusion criteria: Histologically or cytologically confirmed NSCLC. Documented disease progression or lack of clinical benefit on previous treatment with an immune checkpoint inhibitor (PD-1/ PD-L1 inhibitors). Measurable disease as per RECIST version 1.1 criteria. Eastern Cooperative Oncology Group (ECOG) performance status of 0-2. Adequate bone marrow, liver, and renal function. Key exclusion criteria: Prior treatment with anlotinib or the selected chemotherapy agent within the last 6 months. Active brain metastases or leptomeningeal disease. Clinically significant cardiovascular disease within 6 months.
History of interstitial lung disease or pneumonitis. The study protocol and informed consent form have been reviewed and approved by the Institutional Review Board (IRB) or ethics committee at each participating center. Written informed consent will be obtained from all patients prior to enrollment, outlining the study objectives, procedures, potential risks, and benefits.
Gene or biomarker detection
Most of the patients had undergone CT-guided needle aspiration for diagnosis. Once the diagnosis was confirmed pathologically, part of the sample was used for multiple gene detection, such as EGFR, ALK, C-Met, and K-RAS, the detection was performed using standard assay. The detection of PD-L1 was conducted according to the standard assay (Dako 22C-3 antibody), and the cutoff value for PD-L1 was set as 1%.
Lymph cell subpopulation analysis by FCAM
Samples of Ethylenediamine Tetra Acetic Acid (EDTA) anticoagulated peripheral blood (2 mL) were collected from patients with advanced NSCLC before initial treatment and a second sample was collected after subsequent treatment cycles. All samples were tested within 6 hours of being obtained. Briefly, CD3+/CD4+/CD8+ T-cell, CD19+ B-cell, and CD16+CD56+ Natural Killer (NK)-cell counts (cells/μL) were measured by multiple-color flow cytometry with human monoclonal anti-CD3-Fluorescein Isothiocyanate (FITC), anti-CD4-Phycoerythrin (PE), anti-CD8-Allophycocyanin (APC), anti-CD19-PE, anti-CD16-APC, and anti-CD56-PE antibodies [BD Multitest; Becton, Dickinson, and Co. (BD) Biosciences, Franklin Lakes, NJ, USA] according to the manufacturer’s instructions. The cells were analyzed on a BD FACS Canto II flow cytometry system (BD Biosciences).
Treatment Plan
Patients meeting the eligibility criteria will receive anlotinib orally once daily on a 2-weeks-on/1-week-off schedule. The starting dose of anlotinib will be 12 mg/day, with dose adjustments permitted based on individual tolerability and adverse events. Additionally, patients will receive single-agent chemotherapy according to standard dosing and scheduling for their specific disease subtype. Chemotherapy agents may include paclitaxel, docetaxel, gemcitabine, or pemetrexed, chosen based on prior treatment history and disease characteristics.
Assessment of Efficacy and Safety
The primary endpoint of the study is Progression-Free Survival (PFS), defined as the time from initiation of treatment until disease progression or death from any cause. Other endpoints include Overall Survival (OS), Objective Response Rate (ORR) based on RECIST v1.1 criteria, Duration of Response (DoR), and Disease Control Rate (DCR). Safety assessments will include monitoring of Adverse Events (AEs) according to the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI-CTCAE) version 5.0. AEs will be graded for severity and relationship to study treatment. Laboratory evaluations, including complete blood counts, liver and renal function tests, and electrocardiograms, will be performed regularly throughout the treatment period.
Statistical Analysis
The PFS and OS analysis was conducted using SPSS 13.0 (IBM Corp., Chicago, IL, USA), the corresponding figures were drawn using GraphPad Prism 5.0 (GraphPad Software, San Diego, CA, USA). A p value <0.05 was regarded as statistically significant.
RESULTS
Patient Characteristics
The study enrolled a total of 35 patients with advanced NSCLC who had previously progressed following platinum-based chemotherapy and treatment with PD-(L)1 inhibitors. The median age of the cohort was 65 years, The majority of patients had an Eastern Cooperative Oncology Group (ECOG) performance status score of 0-1 (80.00%), with a minority scoring 2 (20.00%). Regarding demographic characteristics, the study population predominantly consisted of males (60.00%), smokers (74.29%), and those with lung adenocarcinoma (68.57%). Additionally, the study included patients with liver metastasis (17.14%), bone metastasis (40.00%), and brain metastasis (22.86%). Furthermore, the expression levels of PD-L1 in enrolled patients were categorized as <1%, 1-49%, and ≥50%, accounting for 57.14%, 28.57%, and 14.29% respectively (See Table 1).
| Characteristics | No. of patients (%) |
|---|---|
| Age | |
| Years | 67 |
| Range | 47-72 |
| Gender | |
| Male | 21(60.00%) |
| Female | 14(40.00%) |
| Smoking history | |
| Never smoker | 9(25.71%) |
| Former smoker | 26(74.29%) |
| Histology | |
| Adenocarcinoma | 24(68.57%) |
| Squamous carcinoma | 11(31.43%) |
| ECOG score | |
| 0–1 | 28(80.00%) |
| ≥ 2 | 7(20.00%) |
| PD-L1 expression level | |
| <1% | 20(57.14%) |
| 1-49% | 10(28.57%) |
| >>50% | 5(14.29%) |
| Previous Radiotherapy | |
| Yes | 5(14.29%) |
| No | 30(85.71%) |
| Brain metastasis | |
| Yes | 8(22.86%) |
| No | 27(77.14%) |
| Bone metastasis | |
| Yes | 14(40.00%) |
| No | 21(60.00%) |
| Liver metastasis | |
| Yes | 6(17.14%) |
| No | 29(82.86%) |
| Stage | |
| IVA | 3(8.57%) |
| IVB | 16(45.71%) |
| IVC | 16(45.71%) |
Efficacy Endpoints
Our preliminary results indicated after the follow up of about 13 months that the combination of anlotinib and single chemotherapy demonstrated significant efficacy, achieving a median progression-free survival and overall survival of 4 months and 8 months, respectively. Kaplan-Meier curves illustrated prolonged PFS and OS in the anlotinib-treated group compared to historical controls, highlighting its potential as an effective treatment option. The Disease control rate, defined by RECIST criteria, was 65%, underscoring the sustained benefit of the combination therapy of Anlotinib and single chemotherapy (See Table 2 and Figure 1).
| Patient No. | Ratio | |
|---|---|---|
| Complete response | 0 | 0 |
| Partial response | 8 | 22.85% (8/35) |
| Stable response | 14 | 40.00% (14/35) |
| Progressive disease | 13 | 37.14% (13/35) |
| Objective response | 22.86% | |
| Median PFS | 4.0 m | |
| Disease control Rate | 65.85% | |
| Median OS | 8.0 m |
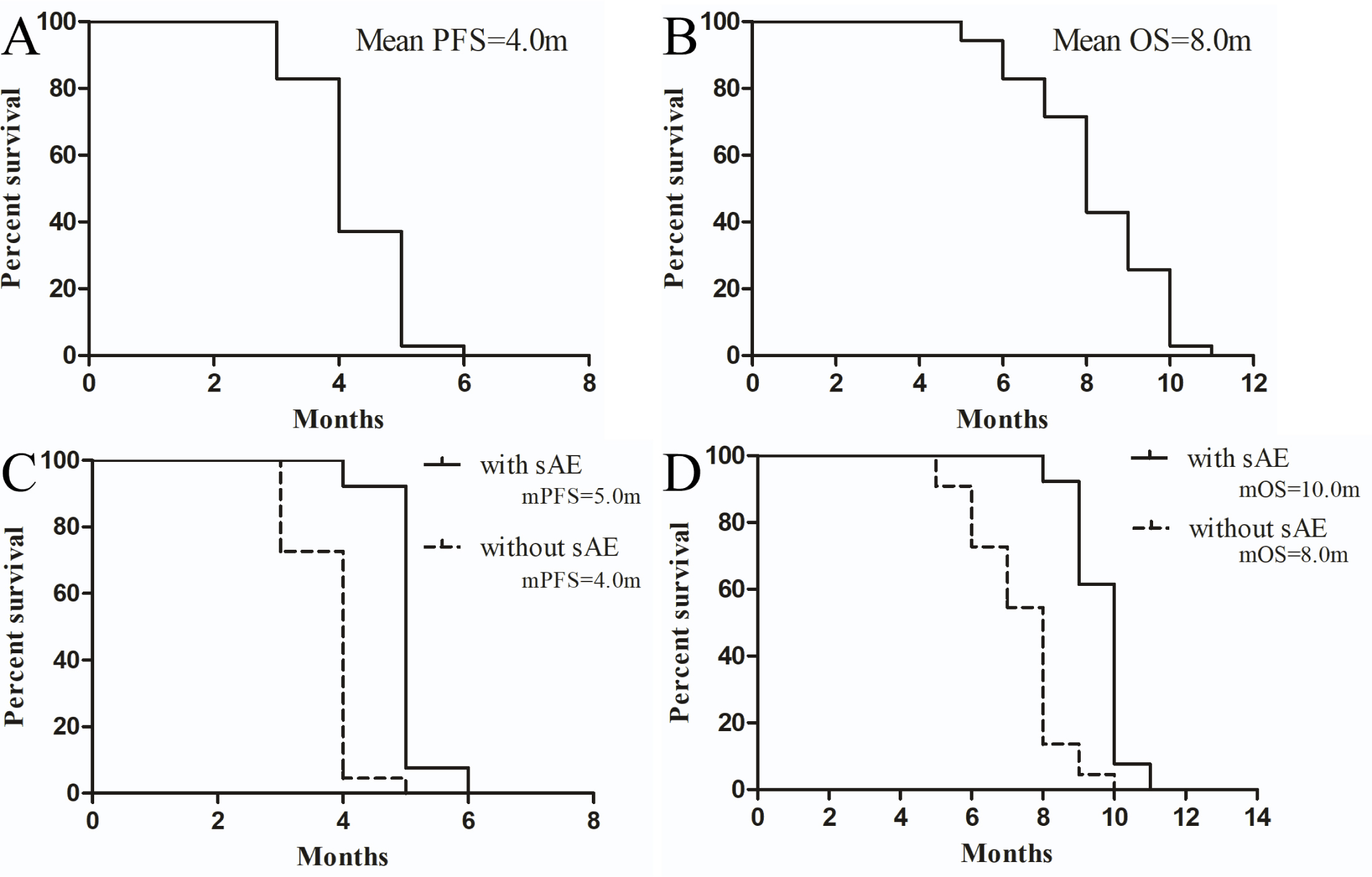
Figure 1:
PFS and OS analysis of general population and subgroup patients with Primary Immune-Resistant NSLC patients who accepted the drug combination of Anlotinib and single Chemotherapy in this study. A and B represent The overall PFS and OS in this study, respectively. C and D represent Comparisons of PFS and OS between these patents with sAEs and without during the whole treatment (with sAEs vs without sAEs) correspondingly. mPFS, median progression-free survival; mOS, median overall survival; sAE, specifically refers to any adverse event including hypertension, proteinuria, and hand-foot syndrome.
Subgroup analysis
To identify advantageous subgroups receiving this regimen, we conducted subgroup analyses. Preliminary findings suggest that patients experiencing specific Adverse Events (sAE) such as hypertension, proteinuria, and hand-foot syndrome during treatment exhibit significantly better efficacy compared to those without toxicities (with sAE vs without sAE, mPFS 5.0 m vs 4.0 m, p<0.0001, HR=0.06508, 95% CI: 0.02139-0.1980; mOS 10.0 m vs 8.0 m, p<0.0001, HR=0.1275, 95% CI: 0.05050-0.3219; See Figure 1). This indicates that certain adverse events may serve as potential predictive biomarkers for the efficacy of this regimen.
Mechanism exploration
To further elucidate potential mechanisms of action and identify potential predictors of therapeutic efficacy, we utilized flow cytometry to assess changes in lymphocyte subsets in peripheral blood of patients before and after treatment. Our study found that patients with advanced Non-Small Cell Lung Cancer (NSCLC) who are primary immune-resistant exhibit higher proportions of Treg cells, while NK cells are generally lower in peripheral blood Compared to the normal reference range, indicating a negative immune microenvironment that contributes to immune therapy resistance. Following three months of treatment with anlotinib combined with single-agent chemotherapy, there was a remarkable decrease in Treg (p=0.009) proportions and an significant increase in NK cell proportions (p<0.001) in peripheral blood of patients (See Figure 2), suggesting a shift from a negative to a positive immune microenvironment. This transformation indicates that the efficacy of this regimen likely operates by reducing or eliminating immune suppression to activate immune function, thereby synergistically enhancing anti-tumor effects.

Figure 2:
The changes in peripheral blood Treg and NK cell proportions in patients with primary immune-resistant NSCLC before and after treatment regimen. A and B respectively represent the changes in peripheral blood Treg and NK cell proportions before and after treatment.
Safety Profile
The combination of Anlotinib and single chemotherapy exhibited a manageable safety profile consistent with its known toxicity profile. The most common treatment-related adverse events included Leukopenia, neutropenia, thrombocytopenia, anemia, fatigue, and decreased appetite, hypertension, hand-foot syndrome, and proteinuria et al., predominantly grades 1-2 in severity, indicating that the toxic side effects of this regimen do not overlap. Serious adverse events, although infrequent, primarily comprised grade 3-4 hypertension and proteinuria necessitating medical intervention (See Table 3). Proactive management strategies, including dose modifications and supportive care, effectively mitigated adverse events without compromising treatment efficacy. Treatment discontinuation due to adverse events occurred in 30% of patients, with disease progression cited as the primary reason for cessation rather than treatment-related toxicity.
| Anlotinib plus CT [n (%)] | ||
|---|---|---|
| Adverse Event | Any Grade | Grade 3 or 4 |
| Hematological | ||
| Leukopenia | 8(22.86%) | 2(5.71%) |
| Neutropenia | 9(25.71%) | 2(5.71%) |
| Thrombocytopenia | 8(22.86%) | 2(5.71%) |
| Anemia | 6(17.14%) | 1(2.86%) |
| Non-Hematological | ||
| Hypertension | 9(25.71%) | 2(5.71%) |
| Hand-foot syndrome | 8(22.86%) | 3(8.57%) |
| proteinuria | 9(25.71%) | 2(5.71%) |
| Elevated transaminase | 5(14.29%) | 1(2.86%) |
| Hyperbilirubinemia | 4(11.43%) | 0% |
| Bleeding | 0% | 0% |
| Fatigue | 12(34.29%) | 0% |
| ALP increased | 2(5.71%) | 0% |
| Elevated GGT | 1(2.86%) | 0% |
| Abdominal pain | 2(5.71%) | 0% |
| Decreased appetite | 14(40.00%) | 0% |
| Hypoproteinemia | 2(5.71%) | 0% |
| Diarrhea | 3(8.57%) | 0% |
| Elevated LDH | 1(2.86%) | 0% |
| Oral ulcer | 2(5.71%) | 0% |
| Stomatitis | 3(8.57%) | 0% |
| Dysphagia | 2(5.71%) | 0% |
| Dysphonia | 1(2.86%) | 0% |
| Rash | 3(8.57%) | 0% |
DISCUSSION
In recent years, Immune Checkpoint Inhibitors (ICIs) have revolutionized the treatment landscape of Non-Small Cell Lung Cancer (NSCLC).[11] However, a subset of patients experiences primary resistance to ICIs or develops resistance after an initial response, posing a significant clinical challenge.[2,12] In such cases, alternative treatment strategies are urgently needed to improve outcomes. in this study, we observed the efficacy and safety of anlotinib combined with single-agent chemotherapy[9] in patients with primary immune resistance in Non-Small Cell Lung Cancer (NSCLC).[13]
The primary endpoint of the study, Progression-Free Survival (PFS) and Overall Survival (OS), serves as a critical measure of treatment efficacy in oncology trials. The observed median PFS and OS of 4.0 months and 8.0 months in our trial exceeded expectations, indicating that the combination of anlotinib with single-agent chemotherapy effectively delayed disease progression in this challenging patient population. This finding is particularly noteworthy given the aggressive nature of immune-resistant NSCLC, underscoring the potential of this treatment approach to provide meaningful clinical benefit.[5,14]
Moreover, Other endpoints such as the Objective Response Rate (ORR) and Disease Control Rate (DCR) further support the efficacy of this regimen. The ORR of 23% and DCR of 65% reflect substantial tumor response and disease stabilization, respectively, highlighting the therapeutic potential of combining anlotinib with chemotherapy.[4] These outcomes are crucial as they suggest not only tumor shrinkage but also sustained disease control, which is essential for improving long-term patient outcomes in advanced NSCLC. Further research has found that its therapeutic efficacy is closely associated with the occurrence of toxic side effects such as hypertension, proteinuria, and hand-foot syndrome, indicating that the occurrence of toxic side effects can serve as potential biomarkers for predicting the efficacy of this regimen.[15,16]
The safety profile of the combination therapy was manageable, with common adverse events including bone marrow suppression, hypertension, fatigue, and proteinuria et al.[17] These adverse events were mostly grade 1 or 2 in severity and were effectively managed through dose adjustments or supportive care measures.[15] Importantly, no treatment-related deaths occurred during the study period, underscoring the safety and tolerability of the anlotinib plus chemotherapy combination in this patient population.[4,7,9] Regular monitoring of hematologic, hepatic, and renal functions facilitated early detection and management of treatment-related toxicities, ensuring that patients received appropriate supportive care throughout the treatment course.[18] This proactive approach to toxicity management is critical in maintaining treatment adherence and optimizing patient quality of life during cancer therapy.[16]
The findings from this study have several important clinical implications for the management of advanced primary immune-resistant NSCLC. Firstly, the efficacy demonstrated by the anlotinib plus chemotherapy combination highlights its potential as a salvage therapy.[19] for patients who have failed or progressed on ICIs. This expands the therapeutic options available to oncologists and provides hope for patients facing limited treatment alternatives.[20,21] Secondly, the safety profile observed in our trial supports the feasibility of combining anlotinib with chemotherapy in clinical practice. With appropriate monitoring and supportive care strategies, clinicians can effectively manage treatment-related adverse events, thereby enhancing patient safety and treatment tolerability.[15] Furthermore, our mechanistic study also revealed for the first time that patients with primary resistance exhibit generally higher proportions of Treg cells and lower proportions of NK cells, indicating a likely negative immune microenvironment in these individuals. This could potentially be a significant factor contributing to immune resistance, especially primary resistance. Following the adoption of combination therapy, however, there was a significant decrease in peripheral blood Treg proportions and an increase in NK cell proportions, suggesting a shift towards a positive immune microenvironment. This indicates that the regimen likely operates by reducing immune suppression to further activate immune responses and enhance synergistic anti-tumor effects.[3] Nevertheless, given the complexity of the immune microenvironment,[22,23] the precise mechanisms of action require further investigation. Looking ahead, future research efforts should focus on elucidating biomarkers predictive of response to anlotinib-based combinations, as well as exploring novel treatment strategies to further improve outcomes in immune-resistant NSCLC, These are also the current focus and direction of the research.[24,25]
CONCLUSION
As far as we know, this is the first study to demonstrate that the combination of anlotinib with single-agent chemotherapy show promising efficacy with manageable safety profile in patients with advanced primary immune-resistant NSCLC, its efficacy is closely associated with certain toxic side effects similar to those of anlotinib, such as hypertension and hand-foot syndrome. The mechanism of action of this regimen likely involves synergistic anti-tumor effects through immune suppression reduction.[24,26,27]
These findings advocate for the exploration of novel treatment combinations beyond conventional immunotherapy approaches, highlighting the importance of personalized medicine in optimizing patient outcomes.[28] Although the preliminary efficacy of the study is promising, there are still some issues with the current research, such as its retrospective nature, the small number of enrolled patients, and the short follow-up period, Further investigation through larger randomized controlled trials is warranted to validate these results and refine treatment strategies in this evolving landscape of lung cancer management.[29,30]
Cite this article:
Li Y, Li W, Liu Y, Peng Y, Tang J, Li X. Efficacy and Safety of Anlotinib Combined with Single-Agent Chemotherapy in Advanced Primary Immune-Resistant NSCLC: A Retrospective Cohort. Journal of BUON. 2024;27:21-7.
ACKNOWLEDGEMENT
We acknowledge the research funding from Natural Science Foundation of Hubei Province (No. 2019CFC929).
The study was supported by Natural Science Foundation of Hubei Province (No. 2019CFC929).
FUNDING
We acknowledge the research funding from Natural Science Foundation of Hubei Province (No. 2019CFC929).
The study was supported by Natural Science Foundation of Hubei Province (No. 2019CFC929).
ABBREVIATIONS
| NSCLC: | Non-small cell lung cancer; |
|---|---|
| PFS: | Progression-free survival; |
| OS: | Overall survival; |
| ORR: | Objective response rate; |
| DCR: | Disease control rate; |
| AEs: | Adverse events; |
| VEGFR: | Vascular endothelial growth factor receptor; |
| PDGFR: | Platelet-derived growth factor receptor; |
| FGFR: | Fibroblast growth factor receptor; |
| ICI: | Immune checkpoint inhibitor; |
| ECOG: | Eastern Cooperative Oncology Group; |
| CT: | Chemotherapy; |
| PD-1/L1: | Programmed death receptor 1/programmed death ligand 1. |
References
- Bade BC, Dela Cruz CS. Lung Cancer 2020: Epidemiology, Etiology, and Prevention. Clinics in chest medicine. 2020;41(1):1-24. [Google Scholar]
- Schoenfeld AJ, Antonia SJ, Awad MM. Clinical definition of acquired resistance to immunotherapy in patients with metastatic non-small-cell lung cancer. Annals of oncology: official journal of the European Society for Medical Oncology. 2021;32(12):1597-607. [Google Scholar]
- Passaro A, Brahmer J, Antonia S, Mok T, Peters S. Managing Resistance to Immune Checkpoint Inhibitors in Lung Cancer: Treatment and Novel Strategies. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2022;40(6):598-610. [Google Scholar]
- Li L, Zhang H, Xie Y. The Efficacy and Safety of Anlotinib Alone and in Combination with Other Drugs in Advanced Lung Cancer: A Retrospective Cohort Study. Computational and mathematical methods in medicine. 2022;2022:1475871 [Google Scholar]
- Li J, Tian Y, Zheng M, Ge J. Anlotinib plus chemotherapy for T790M-negative EGFR-mutant non-sqNSCLC resistant to TKIs: A multicenter phase 1b/2 trial. Thoracic cancer. 2022;13(24):3496-503. [Google Scholar]
- Jiang J, Wu B, Sun Y. Anlotinib reversed resistance to PD-1 inhibitors in recurrent and metastatic head and neck cancers: a real-world retrospective study. Cancer immunology, immunotherapy: CII. 2024;73(10):199 [Google Scholar]
- Xu Q, Huang K, Meng X. Safety and Efficacy of Anlotinib Hydrochloride Plus Temozolomide in Patients with Recurrent Glioblastoma. Clinical cancer research: an official journal of the American Association for Cancer Research. 2023;29(19):3859-66. [Google Scholar]
- Kong T, Chen L, Zhao X. Anlotinib plus etoposide and cisplatin/carboplatin as first-line therapy for extensive-stage small cell lung cancer (ES-SCLC): a single-arm, phase II study. Investigational new drugs. 2022;40(5):1095-105. [Google Scholar]
- Wang HY, Chu JF, Zhao Y. A Trial of the Safety and Efficacy of Chemotherapy Plus Anlotinib vs Chemotherapy Alone as Second- or Third-Line Salvage Treatment for Advanced Non-Small Cell Lung Cancer. Cancer management and research. 2020;12:3827-34. [Google Scholar]
- Yu L, Xu J, Qiao R, Han B, Zhong H, Zhong R, et al. Efficacy and safety of anlotinib combined with PD-1/PD-L1 inhibitors as second-line and subsequent therapy in advanced small-cell lung cancer. Cancer medicine. 2023;12(5):5372-83. [Google Scholar]
- Tang Q, Chen Y, Li X. The role of PD-1/PD-L1 and application of immune-checkpoint inhibitors in human cancers. Frontiers in immunology. 2022;13:964442 [Google Scholar]
- Johnson PC, Gainor JF, Sullivan RJ, Longo DL, Chabner B. Immune Checkpoint Inhibitors – The Need for Innovation. The New England journal of medicine. 2023;388(16):1529-32. [Google Scholar]
- Liu C, Zheng S, Wang Z. KRAS-G12D mutation drives immune suppression and the primary resistance of anti-PD-1/PD-L1 immunotherapy in non-small cell lung cancer. Cancer communications (London, England). 2022;42(9):828-47. [Google Scholar]
- Attili I, Passaro A, de Marinis F. Anti-TIGIT to overcome resistance to immune checkpoint inhibitors in lung cancer: limits and potentials. Annals of oncology: official journal of the European Society for Medical Oncology. 2022;33(2):119-22. [Google Scholar]
- Si X, Zhang L, Wang H. Management of anlotinib-related adverse events in patients with advanced non-small cell lung cancer: Experiences in ALTER-0303. Thoracic cancer. 2019;10(3):551-6. [Google Scholar]
- Li S, Wang H. Research Progress on Mechanism and Management of Adverse Drug Reactions of Anlotinib. Drug design, development and therapy. 2023;17:3429-37. [Google Scholar]
- Cheng JD, Chai LX, Zhao ZP, Hao YY, Li S. Efficacy and Safety of Anlotinib for Patients with Advanced NSCLC Who Progressed After Standard Regimens and the Preliminary Analysis of an Efficacy Predictor. Cancer management and research. 2020;12:5641-50. [Google Scholar]
- Hu H, Chen Y, Tan S. The Research Progress of Antiangiogenic Therapy, Immune Therapy and Tumor Microenvironment. Frontiers in immunology. 2022;13:802846 [Google Scholar]
- Wu K, Fu Y, Gao Z, Jiang J. Salvage therapy of osimertinib plus anlotinib in advanced lung adenocarcinoma with leptomeningeal metastasis: A case report. Respiratory medicine case reports. 2022;38:101682 [Google Scholar]
- Wang P, Fang X, Yin T, Tian H, Yu J, Teng F, et al. Efficacy and Safety of Anti-PD-1 Plus Anlotinib in Patients With Advanced Non-Small-Cell Lung Cancer After Previous Systemic Treatment Failure-A Retrospective Study. Frontiers in oncology. 2021;11:628124 [Google Scholar]
- Dou XJ, Ma RY, Ren DW, Liu Q, Yan P. Effectiveness and Safety of Anlotinib Combined with PD-1 Blockades in Patients with Previously Immunotherapy Treated Advanced Non-Small Cell Lung Cancer: A Retrospective Exploratory Study. Lung Cancer (Auckland, NZ). 2024;15:29-40. [Google Scholar]
- Gao Y, Feng Y, Liu S. Immune-independent acquired resistance to PD-L1 antibody initiated by PD-L1 upregulation via PI3K/AKT signaling can be reversed by anlotinib. Cancer medicine. 2023;12(14):15337-49. [Google Scholar]
- Ricciuti B, Lamberti G, Puchala SR. Genomic and Immunophenotypic Landscape of Acquired Resistance to PD-(L)1 Blockade in Non-Small-Cell Lung Cancer. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2024;42(11):1311-21. [Google Scholar]
- Su Y, Luo B, Lu Y. Anlotinib Induces a T Cell-Inflamed Tumor Microenvironment by Facilitating Vessel Normalization and Enhances the Efficacy of PD-1 Checkpoint Blockade in Neuroblastoma. Clinical cancer research: an official journal of the American Association for Cancer Research. 2022;28(4):793-809. [Google Scholar]
- Luo J, Cheng K, Ji X. Anlotinib enhanced CD8(+) T cell infiltration via induction of CCL5 improves the efficacy of PD-1/PD-L1 blockade therapy in lung cancer. Cancer letters. 2024;591:216892 [Google Scholar]
- Liu S, Qin T, Liu Z. anlotinib alters tumor immune microenvironment by downregulating PD-L1 expression on vascular endothelial cells. Cell death & disease. 2020;11(5):309 [Google Scholar]
- Li X, Peng Y, Wu D, Tang J, Wu Y. Efficacy and safety of anlotinib as maintenance therapy in patients with advanced non-small cell lung cancer achieving SD post first-line chemotherapy combined with immunotherapy. Journal of chemotherapy (Florence, Italy). 2024:1-9. [Google Scholar]
- Yang SR, Schultheis AM, Yu H, Mandelker D, Ladanyi M, Büttner R, et al. Precision medicine in non-small cell lung cancer: Current applications and future directions. Seminars in cancer biology. 2022;84:184-98. [Google Scholar]
- Wang M, Herbst RS, Boshoff C. Toward personalized treatment approaches for non-small-cell lung cancer. Nature medicine. 2021;27(8):1345-56. [Google Scholar]
- Lahiri A, Maji A, Potdar PD. Lung cancer immunotherapy: progress, pitfalls, and promises. Molecular cancer. 2023;22(1):40 [Google Scholar]



